More Information
Submitted: 23 April 2020 | Approved: 29 April 2020 | Published: 30 April 2020
How to cite this article: Luisetto M, Muhamad A, Ibrahim G, Ahmadabadi BN, Khan FA, et al. Evolutive immunologic and toxicologic approach in some neuroinflammatory and degenerative disease like SM, DA, PD: Imaging and Brain Wasting System clearance efficacy. Insights Clin Cell Immunol. 2020; 4: 005-013.
DOI: 10.29328/journal.icci.1001014
ORCiD: orcid.org/0000-0001-8629-0800
Copyright License: © 2020 Luisetto M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Evolutionary; Immunology; Imaging; Neurology; Pathology; Toxicology; New Therapeutic Strategies; DA, PD, ALS
Abbreviations: PD: Parkinson Disease; AD: Alzheimer Disease; CNS: Central Nervous System; MS: Multiple Sclerosys; CSF: Cerebro Spinal Fluid; CA: Contrast Agents
Evolutive immunologic and toxicologic approach in some neuroinflammatory and degenerative disease like SM, DA, PD: Imaging and Brain Wasting System clearance efficacy
Mauro Luisetto1*, Akram Muhamad2, G Ibrahim3, Behzad Nili Ahmadabadi4, Farhan Ahmad Khan5, Ahmed Yesvi Rafa6 and Oleg yurevich latyshev7
1Applied Pharmacologist, Independent Researcher, IMA Academy Pharmacology, Italy
2Department of Eastern Medicine, Government College, Faisalabad University, Pakistan
3Professor, Department of Zoology, Alexandria University, Egypt
4Pharm D/PhD Innovative Pharmaceutical Product Development Specialist, USA
5Professor and Head, Department of Pharmacology Government Medical College Shahdol, Madhya Pradesh, India
6Founder and President, Yugen Research Organizatio, Western Michigan University, MI, USA
7President of IMA, RU, Italy
*Address for Correspondence: Mauro Luisetto, Applied Pharmacologist, Independent Researcher, IMA Academy Pharmacology, Italy, Tel: +393402479620; Email: [email protected]
In order to better understand some neurologic degenerative process is fundamental to use also an evolutionary approach of vertebrates and especially in mammalians. Aim of this work is to verify if an objective measure of brain wasting system can help in this kind of disease. Imaging can help in measuring efficiency of brains wasting system in the various subject. The brain glymphatic systems is well studied today but an accurate measure of the real efficiency of the system is needed. It is relevant so to submit to researcher a working methods strategy to measure this parameter to verify if possible, to use the brain glymphatic system as new therapeutics pathway.
We start this work whit a simply PARADOX: observing that sepia officinal is not show dementia. The different species of animals show various rate of DA dementia and related PD Parkinson disease: It is interesting to observe some literature involved in this topic:
According Pedro J Garcia-Ruiz, et al: There are 2 central premises to this evolutionary view of Parkinson- disease PD. First, PD is a specific human disease. Second, the prevalence of PD has increased over the course of human history. Several lines of evidence may explain why PD appears to be restricted to the human species. The major manifestations of PD are the consequence of degeneration in the dopamine-synthesizing neurons of the meso-striatal neuronal- pathway. It is of note the enormous expansion of the human dopamine mesencephalic neurons onto the striatum compared with other mammals. Hence, an evolutionary bottle neck was reached with the expansion of the massive nigrostriatal axonal- arborization. This peculiar nigral overload may partly explain the selective fragility of the human dopaminergic mesencephalic neuro-transmission and the unique presence of PD in humans. On the other hand, several facts may explain the increasing prevalence of PD over the centuries. The apparently low prevalence of PD before the twentieth century may be related to the shorter life expectancy and survival compared to present times. Changes in lifestyle over the course of human -history might also account for the increasing burden of PD. Our hunter gatherers ancestors invested large energy expenditure on a daily basis, a prototypical physical way of life for which our genome remains adapted. Technological -advances have led to a dramatic reduction of physical exercise. Since the brain release of neuro-trophic factors (including brain-derived neuro-trophic factor) is partially exercise related, the marked reduction in exercise may contribute to the increasing prevalence of PD. Many neurological- diseases can be found in non-human mammals both acquired and hereditary (such as myelopathy, brain tumors, epilepsy, muscular dystrophy, and narcolepsy, to mention a few). AD and PD are considered specific to Homo sapiens. While there are useful animal models of PD including MPTP and alpha-synuclein-over-expressing transgenic mouse- models, which may recapitulate important clinical features of the human disorders, especially in aged monkeys, no spontaneous akinetic-rigid syndrome is known to occur in wild- mammals including non-human primates.
Vernier P, et al: PD is to a large extent, specific to the human species. Most symptoms are the consequence of the preferential degeneration of the dopamine-synthesizing cells of the meso-striatal-meso-cortical neuronal pathway. Reasons for that can be traced back to the evolutionary- mechanisms that shaped the dopamine neurons in humans. In vertebrates, dopamine-containing neurons and nuclei do not exhibit homogenous phenotypes. In this respect, mesencephalic dopamine neurons of the substantia nigra and ventral- tegmental area are characterized by a molecular combination (tyrosine hydroxylase, aromatic amino acid de-carboxylase, mono-amine oxidase, vesicular mono-amine transporter, dopamine transporter--to name a few), which is not found in other dopamine-containing neurons of the vertebrate brain. the size of these mesencephalic DA nuclei is tremendously expanded in humans as compared to other vertebrates. Differentiation of the mesencephalic neurons during development depends on genetic- mechanisms, which also differ from those of other dopamine nuclei. pathophysiological approaches to PD have highlighted the role of ubiquitously expressed molecules such as a-synuclein, parkin, and microtubule-associated proteins. We propose that the peculiar phenotype of the dopamine mesencephalic neurons, which has been selected during vertebrate -evolution and reshaped in the human lineage, has also rendered these neurons particularly prone to oxidative- stress, and thus, to the fairly specific neuro-degeneration of PD. evidence demonstrate that perturbed regulation of DAT-dependent dopamine uptake, DAT-dependent accumulation of toxins, dysregulation of TH activity as well as high sensitivity of DA mesencephalic neurons to oxidants are key components of the neuro-degeneration process of PD. This view points to the contribution of non-specific -mechanisms (alpha-synuclein aggregation) in a highly specific cellular environment (the dopamine mesencephalic neurons) and provides a robust- framework to develop novel and rational therapeutic schemes in PD.
So evolutive patterns of mammalian are relevant fact to be taken in consideration in some neurodegenerative disease like PD. In some neurological conditions is clear the role played by brain immune system and the global effect Due by BEE and other factor like brain washing system. Observing the complex- mechanism involved in KURU disease is possible to verify the role played by Specific and A-specific immune system. (Innate and Adaptative). The same observing the anatomy/physiology of CNS, BEE and relate immune system: role played by astrocyte, microglia and other cells. The evolutionary approach makes possible to verify evolution of CNS, BEE and related brain structure and the related selective- vulnerability. Form invertebrates to superior vertebrate’s various physio-anatomical modifications emerged. The new need in movement and in environmental relationship request more complicated structure and the same from primitive- invertebrates to the more complex superior vertebrates the same immune system requested new strategy. Also regenerative of retina show great difference in neuronal- evolution in the various species. Neocortical cognitive activities needed a new sovra-structure development.
Archeo brain vs. neo Brain
But this sovra-structure was moves to produce circon-voluntion in cortex to increase number of neurons. But the same this created some wasting -system increased problems? Why in example cerebellum is later interested by PD vs basal ganglia? Different neurodegenerative disease present different neuronal toxicity. Superior vertebrates need less regenerative- properties then more primitive organism. And if DA affect more cognitive cortex PD the extrapyramidal first. Some characteristic of brain evolution with new sovra-structures in old ones created vulnerability. Some question is really interesting: What is the role played by the fact that CNS is a kind of sancturaries? And the fact that loss of A complete lymphatic system? no limpho-nodes inside. And the role -played by BEE even in the sense of from inside to outside barrier for toxic molecule? Why in brain white matter is outside vs grey matter and opposite in spinal cord? Is due to the progressive increase of cortical surface? Why cervical region is so often involved in some neuro-degenerative pathology? There is a specific vulnerability? and why in example cerebellum is not involved in initial stages of PD? What are the role played by endogenous toxic- movens? And how is possible to depurate this kind of toxic condition?
Aim of this work is to verify if Imaging technique can help in PD therapeutic strategy, applied in a wide range of patients in order to verify brain wasting system efficacy and if it is clinically relevant as hypotetic way to depurate it from the toxic molecule usually find in this kind patients.
With an observational approach some relevant biomedical literature are analyzed to produce a global conclusion related the topics of this work. Al literature presented is presented in scientific biomedical database like PUBMED [1-49] or Other open science journals. Most of the literature analyzed is related last year and so recent. After this review a global conclusion is produced related to the topics of this work. In the last phases an experimental hyoptesys of work to be verified is submitted to the researcher for other new study and in order to verify it.
From literature
We have seen from scientific literature the relationship existing between systemic immune status and local situation like in brain tissue. We can consider under a toxicological view this kind of influences in order to re- consider some brain pathologies especially if time age related. (Peaks-age classes more involved in some neurologic- pathologies). Local flogosys and related immune reaction activation contribute in some brain- pathology and this can be considering a sort of toxicological effect that must to be deeply investigated in order to discover the pathogenetic moves and innovative pharmacological strategies. Toxicology science can add to immunology - pathology to have a more complete vision in some brain pathology in time evolution and strategic opportunities. We have seen in example that using fingolimod we have a reduction in linfocites activation and when discontinued this effect reduced (like a discontinue of a toxic- substantia). Dose related and time related. Significantly improved relapse- rates and end points measured on magnetic resonance imaging in objective -way. Concepts as toxical doses, time of exposition, cumulative dosage, kinetics, dynamics, metabolism Iatrogenic ADME and other toxicological parameter can be usefully introduced also in neuro-immune toxicology to adequately focus a physio-pathogenetic phenomena. The results related to the references cited show a specific- effect of a systemic drugs in a local place as brain. So- observing a specific side- effect of a drug can be a right method to clear some interference between the immunologic status and some development disorder [1].
Related to the reference findings presented in this review and research- work is crucial to submit to the researcher a new hypotesis related the anatomic decussatio pyramid and cervical tract in global physio-metabolic status of Spinal cord. In same condition like repeated head micro-trauma, or in particular weakness of motor -neuron this anatomic conformation can produce un unbalance that can aggravate this situation. (See the higher frequencies related cervical tract vs other in spinal cord). A fact to be take in consideration even if is a normal anatomic- conformation. The experimental project proposed make possible to verify any connection between an anatomic- peculiarity (decussation pyramidi, cervical spinal- cord tract) and an increased weakness of the neuronal tracts. So in neuro- degenerative spinal cord disease is relevant to observe the global- topography of the lesions to verify if some tract are more involved vs other to better understand the real reason. The evolutionary approach make possible to better understand the global- process under new light [2].
Related the result of this work is relevant for some neuro-degeneretaive condition to verify the role played by brain glymphatic -system as well as the effect played by some brain vascular inefficiencies and the role played by SOME TOXIC catabolic substantia and their accumulation. An unbalance between production and clearance of alfa sinuclein in the brain is involved in PD evolution. The relationship with some kind of food and PD is not so high but what is to take in consideration is the functionality of the brain clearance -system that can be more saturated is some kind of diet. Also interesting the way of influence in this system by body- posture (is a dynamic -process). Is possible that 2 factors influence this process?
Factor A: clearance -efficiency of the system in basal status (genetic factor, age)
Factor B: Clearance -efficiency in SATUTARED situation? (Environmental -factor) and
Global function: A x B
This result can be used to search new therapeutic strategies: or to reduce saturation condition of the system analyzed or new molecule that can improve the “washing” of this toxic endogenous substantial or a combination of this 2. A Detox- gel in mouse model of DA showed activity to suggest to develop this strategy, and the same observing epidemiological data related world PD incidence compared to some diet it seen to show how reduce the saturation of this system. As global conclusion of this work is possible to say that a combination of these 2 strategies can produce an interesting clinical- effect to be verified. (This combined- strategy is a new- association: Pharmacological effect, added to de toxicant strategy). Four are the main factor involved: The toxic molecule to be depurate, the carrier- vectors, the brain washing system, saturation condition (Environmental factor). In animal model the detox strategy produced effect, so is possible to say that a toxicological approach can be a way to be walked. A “total Aβ-42 by 30% in the group of mice that received the detox -gel when compared to the untreated group with a statistical significance (p < 0.001)” (5) is a good result to start. A new drug class able to link and detox the toxicological- molecule and to be carried out from brain- structure using the glimphatic system through adequate pharmaco-kinetics chemical group. So in drug design activity: Drugs with 2 parts: A part that link the toxico molecule (de-tox), added to another part with Pharmacokinetics group for brain glimphatic system. Molecule when the necessary bio-tolerability, absence of toxicity and high kinetics properties (to arrive in site of actions and to be washed). High affinity, sequestering ability, not immunogenicity, right molecular weight, lipofilic idrofilic balances right electrical charges, persistence of actions and other relevant molecular properties drive in the research of this new drugs. BEE is a crucial protective- structure of CNS since from outside of it but it can be also crucial in the outside. Transfer of toxic- catabolic products. A deep knowledge in this dynamic make possible to search innovative approach in an endogen- toxicological condition. Keyword to be investigated: brain wasting -molecule clearance, kinetics of the process, genetic, environmental- factors, saturation process. The finding in animal model are interesting point to start [3] (Figures 1-4).
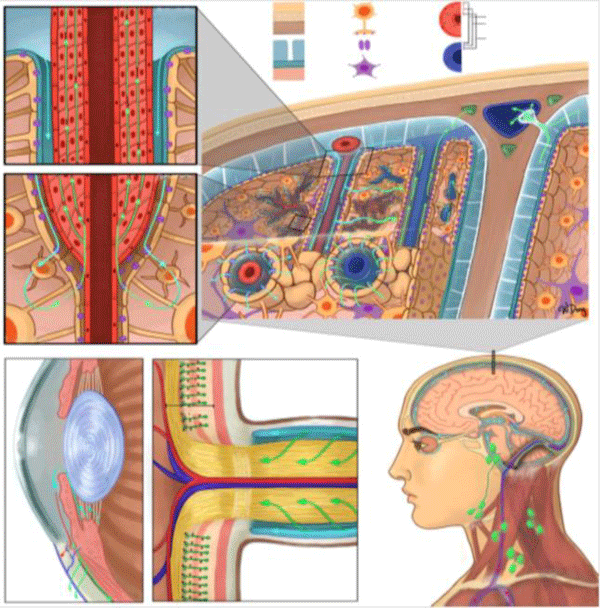
Figure 1: Schematic of major clearance systems in (A,B,C,F) the brain and (D,E,F) the eye. (A,B,C) are representations of the IPAD and glymphatic pathways; (A) is a cross-section of an arteriole and represents CSF flow (cyan arrows) from the SAS into the peri-arterial space, as well as ISF flow (green- arrows) through the smooth muscle- basement membranes; (B) is a cross-section of an arteriole transitioning into a capillary, where CSF exits the peri-arterial space via AQP4 water- channels (purple) located on the astrocytic endfeet before mixing with ISF (cyan green arrow) and entering the smooth muscle basement -membranes; (C) is a coronal cross-section through the head and represents the glymphatic -pathway, dorsal mLVs, and CSF flow through an AG. CSF flows from the SAS into peri-arterial spaces before flowing intothe brain parenchyma via AQP4 channels, mixing with ISF, and then entering the perivenous- space for drainage via a convective flow.
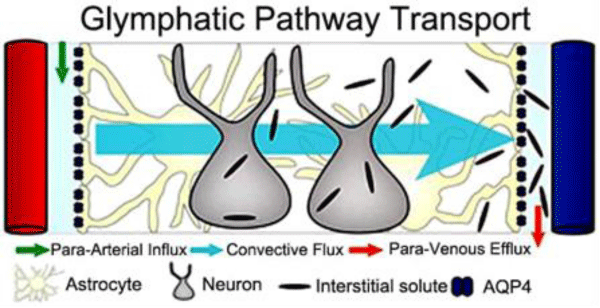
Figure 2: Principle of glymphatic transport in rodent brain. Peri-arterial inflow of cerebrospinal fluid (CSF) enters the brain tissue facilitated by astrocytic end feet AQP4 water- channels; mixes with interstitial fluid and removes the waste- products into peri-venous space.
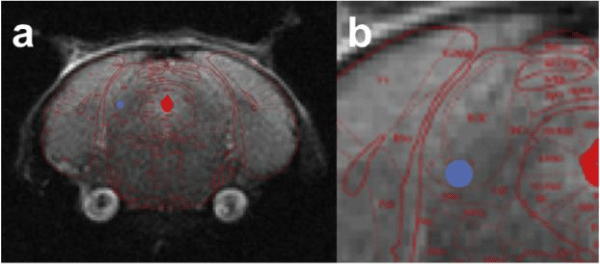
Figure 3: Insertion location of electrode in right hemipshere. a) Whole brain coverage of location for probe insertion. b)Simulated probe location (AP = -4.70 mm Bregma, ML = 1.50 mm and DV = -2.50 mm Bregma).
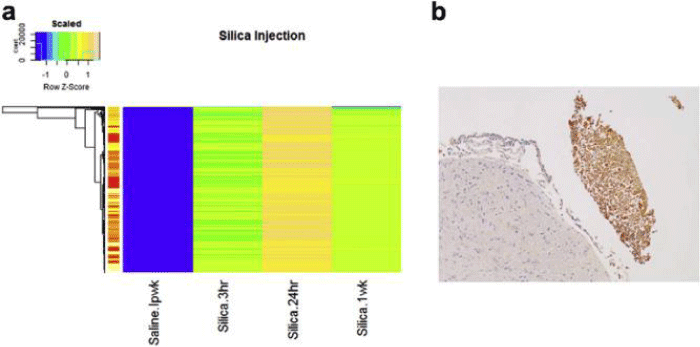
Figure 4: Heatmap of LIBS spectrum results from simulated silicon probe insertion and isolated immune response. a) Heatmap differentiating control (saline group), and silicon- groups (3hrs, 24hrs, and 1 wk). The scaled difference is -1 to 1 with silicon 24hr being most different from the control. The immune response at 24hr likely contributed to the greatest difference in the spectra. b) Isolated immune- response was found by IHC macrophage staining of CD68 (AB31630). Note the response was isolated and near the inferior- colliculus injection site, but located exterior to the needle -mark.
Observing that some cases of CJD are due by peripheral- exposition to prions and that KURU- disease and BSE are transmitted by oral intake of infected food we try to produce new theories in immune systems and brain inter-connections. Can we consider KURU- infectious disease an instrument to verify inter-connection between immune cell out and in central nervous systems? Like a RLIC magic bullet? Or an imaging tracer to follow the neuro immune -process? Prions result neurotropic but other antigen are normally presented inside brain? And with what consequences? Other brain disease presents the similar patho-genetic move’s immune system related? Transgenic modified mouses study showed that immune system is involved in amplification and transmission to CNS (Lymph. B and follicular dendritic cell). In prions disease we see species barrier and is necessary a molecular similarity between prions and endogenous PRP-C. Is present relationship between some brain disease as amyloidosis and other degenerative- disease like PD, DA and prions disease and other? In SM (a neuro inflammatory pathology) is involved adaptative- immunity: Lymphocyte T and B, while in other disease as Alzheimer (a neuro-degenerative pathology) is involved the innate immunity (microglia- macrophage like activity, first immune- control system in the CNS). Observing the KURU disease, the time involved in presentation of symptomatology after intake of Prions and (a slow process) related to the fast time In some cases involved in some neurotropic viruses we can think to an passive vs active process by which immune systems transfer to the brain the toxic prions from GI system. In all this pathology immune system play a relevant role (adaptive or innate) giving tissue damage and accumulation of bio- products. Observing the global role played by immune system in some brain pathology under a specific toxicological- aspect We can think to other therapeutic strategies to improve the actual pharmacological- scenario. This paper is produces under a specific medicinal chemistry and pharmacological point of view [4].
Taoka T, et al: The existence of a mass transport system in the brain via cerebrospinal fluid (CSF) or interstitial fluid (ISF) has been suggested by many studies the glymphatic -system is hypothesized to be a waste clearance system of the CSF through the perivascular and interstitial spaces in the brain. Tracer studies have primarily been used to visualize or evaluate the waste clearance system in the brain, and evidence for this system has accumulated. The initial- study that identified the glymphatic system was an in vivo tracer study in mice. In that study, fluorescent -tracers were injected into the cisterna- magna and visualized by 2-photon microscopy. MRI has also been used to evaluate glymphatic function primarily with gadolinium-based contrast agents (GBCAs) as tracers. A number of GBCA studies evaluating glymphatic- function have been conducted using either intrathecal or intravenous- injections. Stable isotopes, such as 17 O-labeled water, may also be used as tracers since they can be detected by MRI. In addition to tracer studies, several other approaches have been used to evaluate ISF- dynamics within the brain, including diffusion imaging. Phase contrast evaluation is a powerful method for visualizing flow within the CSF space. In order to evaluate the movement of water within tissue, diffusion-weighted MRI represents another promising technique, and many studies have utilized diffusion- techniques for the evaluation of the glymphatic -system. This work will discuss the findings of these diffusion studies [5].
Davoodi-Bojd E, et al: The glymphatic system is functional waste clearance path from the brain parenchyma through dynamic exchange of cerebrospinal fluid with interstitial fluid (ISF). Impairment of glymphatic waste clearance is involved in the development of neurodegenerative conditions. Despite many recent studies investigating the glymphatic system, few studies have tried to use a mathematical model to describe this system, quantitatively. In this work, we aim to model the glymphatic- system from the kinetics of Gd-DTPA tracer measured using MRI in order to: 1) map the glymphatic- system path, 2) derive kinetic parameters of the glymphatic system, and 3) provide quantitative- maps of the structure and function of this system. In the proposed model, the brain is clustered to similar regions with respect to the profile of contrast agent (CA) density measured by MRI. Then, each region is described as a 2-compartment kinetic model ‘derived from’ or ‘clears to’ its neighbors with local- input function. We thus fit our model to the local cerebral regions rather than to the averaged time signal curve (TSC) of the whole brain. The estimated parameters showed distinctive differences between diabetes- mellitus and control rats. The results suggest that in a typical DM brain the CSF bulk speed in the para-vasculature network is low. In addition, the resulting maps indicate that there may be increased binding and decreased absorbing of large -molecules in a diabetic compared with a non-diabetic brain. The important contribution of this work was to fit the model to the local- regions rather than to the averaged time signal curve (TSC) of the whole brain. This enabled us to derive quantitative maps of the glymphatic- system from MRI [6].
Kristian eide, et al: Pre-clinical research in rodents provides evidence that the CNS has functional lymphatic vessels. In-vivo observations in humans, however, are not demonstrated. We here show data on CNS lymphatic drainage to cervical lymph nodes in vivo by magnetic resonance imaging (MRI) enhanced with an intra-thecal contrast agent as a cerebrospinal fluid (CSF) tracer. Standardized MRI of the intracranial compartment and the neck were acquired before and up to 24–48 hours following intra-thecal contrast agent administration in 19 individuals. Contrast enhancement was radiologically confirmed by signal changes in CSF nearby inferior frontal gyrus, brain parenchyma of inferior frontal gyrus, para-hippocampal gyrus, thalamus and pons, and parenchyma of cervical lymph node, and with sagittal sinus and neck muscle serving as reference tissue for cranial and neck MRI acquisitions, respectively. Time series of changes in signal- intensity shows that contrast- enhancement within CSF precedes glymphatic- enhancement and peaks at 4–6 hours following intra-thecal injection. Cervical lymph node enhancement coincides in time with peak glymphatic enhancement, with peak after 24 hours. Our findings provide in-vivo evidence of CSF- tracer drainage to cervical lymph nodes in humans. The time course of lymph node enhancement coincided with brain- glymphatic enhancement rather than with CSF- enhancement [7].
Wenyu Deng, et al: Debilitating neuro-degenerative conditions, such as MS, AD and PD, are often presented with the accumulation of metabolic by-products in brain tissues. Studies also suggest linkages between ocular and cerebral diseases, yet the underlying mechanisms remain unclear. Unlike the rest of the body, the CNS does not comprise lymphatic- vasculature for metabolic waste removal. Instead, several hypotheses have been proposed that rely on the complex but highly- regulated clearance mechanisms responsible for an adequate neurona-l environment and fluid homeostasis. Understanding the mechanisms of clearance systems in the eye and the brain can help exploit fluid transport and potentially offer new targets for therapy to the visual- system and beyond. we describe and criticize how quantitative- imaging can play a role in evaluating different models of clearance systems. Challenges in imaging the clearance systems of the eye and the brain. Apart from the physical- factors from imaging techniques in probing the mechanisms of clearance systems, CNS- waste clearance appears to be affected by a number of physiological factors such as wakefulness, anesthesia regimes, exercise, age, tracer delivery, and posture. CSF tracer influx appears to be suppressed in awake subjects, increased after voluntary- exercise, and increased or decreased in sedated subjects depending on the anesthesia regimes. at a high anesthetic dose such as 3% isoflurane, general anesthesia may have a negative-impact on the intracranial CSF circulation. This occurs not simply by inducing un-consciousness but also by additional mechanisms including repression of nor-epinephrine release. To minimize the effects of anesthesia on solute transport during imaging, anesthesia with dexmedetomidine and low-dose isoflurane has been proposed if awake imaging is not feasible.
CNS waste clearance research often requires surgical procedures for the administration of tracers prior to image acquisition. In animal research studies, imaging of lymphatic drainage in the eye typically relies on intra-cameral, intra-vitreal, or subretinal contrast injection. For imaging brain waste clearance, intra-thecal catheterization is desirable over intra-cranial administration since it eliminates the need for a cranio-tomy. SAS catheterization via the atlanto-occipital membrane allows catheter insertion into the cisterna- magna or down to the lower levels of the lumbar spinal cord of rats. Longitudinal studies in rodents typically rely on lumbar intra-thecal catheterization for the infusion of tracers into the CSF. MRI studies of human glymphatic function also rely on lumbar- puncture and intrathecal- administration of CSF tracers to ensure brain-wide CSF contrast enhancement and clearance. Intra-cerebro-ventricular tracer- injection can also be used, yet cautions should be noted when performing invasive -procedures for glymphatic studies, as it is reported that intra-striatal injections suppress glymphatic function. Quantitation of the clearance systems in the eye and the brain.
Quantification of the complete CNS waste-clearance system remains challenging partly due to limitations in tracer choices for full eye or brain in vivo research, and in the resolution and specificity of non-invasive imaging- techniques. indirect -measurements in distal locations, synchronization with physiological monitoring and biophysica-l modeling may help improve the understanding of the relative contributions of individual components to the clearance -systems. upon intraocular injection of radioactive- tracers, we can quantify the clearance in the trabecular meshwork and uveo-scleral pathway separately by measuring the time progression of total radioactivity in the plasma and lymphatic tissues, respectively using a gamma counter. Quantification of CSF flow can be accomplished with fluorescent- tracers and microspheres infused into the cisterna- magna of mice and visualized by 2-photon microscopy, while simultaneously measuring pulse and respiration. Microsphere velocity measurements, electro-cardiograms, and spectral analyses demonstrate that CSF flow more closely resembles the cardiac cycle than the respiratory cycle, suggesting that the primary driver of CSF flow is perivascular pumping. The synchronization of diffusion-weighted MRI acquisition with electro-cardiogram also allows for the characterization of the effect of arterial pulsatility on the perivascular space and the surrounding fluid -movement in terms of pseudo-diffusivity indices.
In terms of biophysical modeling, a 2-compartment pharmacokinetic model for GBCA-based glymphatic transport has been tested in rats with severe disruption in micro- and macro-vasculature induced by diabetes mellitus. The contrast concentration in 2 compartments (free and bound fractions) is represented by a system of differential equations describing contrast dynamics among the arterial peri-vasculature and brain parenchyma. The model solution has a bi-exponential form whose respective fractions and time constants allow the estimation of contrast retention and loss to the perivascular -space. This approach has also been used to demonstrate the dependence of glymphatic-- clearance on corporal- position. Experiments showed reduced overall clearance in rats scanned in a prone position compared to supine or right lateral decubitus positions. These results were verified with fluorescence and radio-labeled tracer imaging, showing comparatively greater CSF influx in the supine and right lateral decubitus positions. The spontaneous rhythmic oscillations of vascular tone in the beds of various tissues, including the cerebral tissues, known as vasomotion, are central to the IPAD model for brain- waste clearance. This model encompasses a complex system of equations, representing vasomotion-induced intramural peri-arterial flow through the poro-elastic basement membrane, coupled with the elastic response of the middle cerebral artery in the arterial wall model. This model has been proposed to explain the mechanism of IPAD- pathways through the basement- membranes. [10]
Schematic of major clearance systems in (A,B,C,F) the brain and (D,E,F) the eye. (A,B,C) are representations of the IPAD and glymphatic pathways; (A) is a cross-section of an arteriole and represents CSF flow (cyan arrows) from the SAS into the peri-arterial space, as well as ISF flow (green- arrows) through the smooth muscle- basement membranes; (B) is a cross-section of an arteriole transitioning into a capillary, where CSF exits the peri-arterial space via AQP4 water- channels (purple) located on the astrocytic end feet before mixing with ISF (cyan green arrow) and entering the smooth muscle basement -membranes; (C) is a coronal cross-section through the head and represents the glymphatic -pathway, dorsal mLVs, and CSF flow through an AG. CSF flows from the SAS into peri-arterial spaces before flowing intothe brain parenchyma via AQP4 channels, mixing with ISF, and then entering the perivenous- space for drainage via a convective flow. Fluid from the SAS can then drain into the mLVs (green openings) surrounding the SSS; (D) represents a cross-section of the anterior chamber of the eye. Cyan- arrows represent production and flow of aqueous humor from the ciliary body. Red arrow- represents the trabecular meshwork pathway where aqueous humor- flows into the episcleral vein by passing through the Schlemm’s canal (blue opening). Green arrow represents the uveoscleral pathway where aqueous humor flows through the interstitial trabeculae of the ciliary bodies and enters the supra-choroidal space; (E) represents a cross-section of the optic nerve head. Müller cells within the retina, which share similar functions to astrocytes in the brain, are represented in dark green, with appendages that wrap around retinal- capillaries (red dots), constituting part of the blood-retinal barrier. The broad stripe behind the Müller -cells represents the INL. The optic- nerve is surrounded by SAS through which CSF flows into the optic nerve; (F) is a diagram of CSF- flow within the SAS, originating from the choroid plexus (orange) within the ventricles, as well as dorsal and basal mLVs. These mLVs travelling alongside the TS and SS, exiting out of the jugular -foramen with the internal- jugular vein, and draining into the deep- cervical lymph nodes. Pre-auricular [1], submandibular [2], superficial cervical [3], deep cervical [4], and supraclavicular [5] lymph nodes are illustrated. IPAD, intra-mural periarterial drainage; CSF, cerebrospinal fluid; SAS, subarachnoid space; ISF, interstitial fluid; AQP4, aquaporin-4; mLVs, meningeal lymphatic vessels; AG, arachnoid granulation; SSS, superior sagittal sinus; INL, inner nuclear layer; TS, transverse sinuses; SS, sigmoid sinuses.
According Muhammad Shehzad Khan: “Silicon-based devices, such as neural probes, are increasingly used as electrodes for receiving electrical signals from neural tissue. Neural probes used chronically have been known to induce inflammation and elicit an immune response. The current study detects and evaluates silicon dispersion from a concentrated source in the mouse brain using laser induced breakdown spectroscopy. Element lines for Si (I) were found at the injection site at approximately 288 nm at 3hr post-implantation, even with tissue perfusion, indicating possible infusion into neural tissue. At 24hr and 1-week post-implantation, no silicon lines were found, indicating clearance. An isolated immune response was found by CD68 macrophage response at 24hr post injection. Future studies should measure chronic silicon exposure to determine if the inflammatory response is proportional to silicon administration. The present type of protocol, coupling laser induced breakdown spectroscopy, neuroimaging, histology, immunohistochemistry, and determination of clearance could be used to investigate the glymphatic system and different tissue states such as in disease (e.g. Alzheimer’s) [50].
Experimental hypotesys project
In order to test single individual efficience of brain glimphatic system is need to obtain basal level of normal individuals (animal model? population data). It is necessary to use a right treaceant medium for imaging scope and verify kinetics. Data organized by age and other parameters.
Related all literature reported in reference and in the text is possible to verify that: a)“AD and PD are considered specific to Homo sapiens” GARZIA RUIZ, b)“In animal model the detox strategy produced effect, so is possible to say that a toxicological approach can be a way to be walked. A “total Aβ-42 by 30% in the group of mice that received the detox gel when compared to the untreated group with a statistical significance (p < 0.001)” (5) is a good result to start. A new drug class able to link and detox the toxicological molecule and to be carried out from brain structure using the glimphatic system through adequate pharmacokinetics chemical group [3], c) “CNS waste clearance appears to be affected by a number of physiological factors such as wakefulness, anesthesia regimes, exercise, age, tracer delivery, and posture. CSF tracer influx appears to be suppressed in awake subjects, increased after voluntary exercise, and increased or decreased in sedated subjects depending on the anesthesia regimes, d) “The glymphatic system is functional waste clearance path from the brain-- parenchyma through dynamic exchange of cerebrospinal fluid with interstitial fluid (ISF). Impairment of glymphatic waste clearance is involved in the development of neurodegenerative- conditions, e)”Our findings provide in-vivo evidence of CSF- tracer drainage to cervical lymph nodes in humans, f) “Apart from the physical- factors from imaging techniques in probing the mechanisms of clearance systems, CNS waste clearance appears to be affected by a number of physiological factors such as wakefulness, anesthesia regimes, exercise, age, tracer delivery, and posture. CSF tracer influx appears to be suppressed in awake subjects, increased after voluntary exercise, and increased or decreased in sedated subjects depending on the anesthesia regimes.
Related the topics of this works observing the literature reported and other in biomedical database is clear that imaging new technique are needed to adequately evaluate the brain washing system in order to verify In example individual clearance efficacy towards various toxic movens (alfa synuclein, neuronal cell inclusion, free radicals, immune molecule and other). In some mouse model Glymphatic clearance of simulated silicon dispersion (iatrogenic) was evalued and the same other brain clearance must be measured. In all literature observed there is no a systematic measure of this parameter in groups of patients’ with neurodegenegerative of neuroinflamatory disease. The same in populations of subject to evaluate the prevalence of clearance disfunctions. A better quantitative measure of this clearance makes possible to find new therapeutic strategies.
- Luisetto M. Brain response in some systemic immune condition-Toxicological aspects. Insights Clin Cell Immunol. 2017; 1: 5-8.
- Luisetto M, Farhan AK, Ahmed YR, Behzad NA, Ghulam RM. Decussatio Pyramid and Optical Chiasm as an Interesting Example of Evolutionary Process Useful in Understanding Some Spinal Cord Phenomena? On J Neur & Br Disord. 2019; 2.
- Luisetto M, Khan FA, Muhamad A, Mashori GR, Ahmadabadi BN, et al. Brain washing systems and other circulating factors in some neurological condition like Parkinson (Pd) and vascular and diabetic dementia: How dynamics- saturation of clearance can act on toxic molecule? J Neurosci Neurol Disord. 2020; 4: 1-13.
- Luisetto M, et al. Brain and Immune System: KURU, a Strange Kind of Disease. An Endogenous Toxicological Process Like? EC Neurology 2018; 10: 7.
- Taoka T1, Naganawa S1. Glymphatic imaging using MRI. J Magn Reson Imaging. 2020; 51: 11-24. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31423710
- Davoodi BE1, Ding G, Zhang L, Li Q, Li L, et al. Modeling glymphatic system of the brain using MRI. Neuroimage. 2019; 188: 616-627. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30578928
- Per KE, Svein SV, Kyrre EE, Geir R. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes Sci Rep. 2018; 8: 7194. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29740121
- Pedro JGR, Alberto J, Espay. Parkinson Disease: An Evolutionary Perspective image. Front Neurol. 2017.
- Vernier P1, Moret F, Callier S, Snapyan M, Wersinger C, et al. The degeneration of dopamine neurons in Parkinson's disease: insights from embryology and evolution of the mesostriatocortical system. Acad Sci. 2004; 1035: 231-249.
- Wenyu D, Crystal L, Carlos P, Jeffrey R. Muneeb A, et al. Quantitative imaging of the clearance systems in the eye and the brain. Quant Imaging Med Surg. 2020; 10: 1-14. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31956524
- Abbott NJ. Blood–brain barrier structure and function and the challenges for CNS drug delivery. Journal of inherited metabolic disease. 2013; 36: 437-449.
- Abbott NJ. Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiology of disease. 2010; 37, 13-25.
- Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Molecular neurodegeneration. 2016; 11: 74.
- Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids and Barriers of the CNS, 2014; 11: 10. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24817998
- Damkier HH Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiological reviews. 2013; 93: 1847-1892.
- Davson H, Segal MB. Physiology of the CSF and blood-brain barriers. Boca Raton. 1996; 1.
- Del Zoppo GJ, Moskowitz M, Nedergaard M. The neurovascular unit and responses to ischemia. Elsevier. 2016; 90-101.
- Di Terlizzi R, Platt S. The function, composition and analysis of cerebrospinal fluid in companion animals: Part I–Function and composition. The Veterinary Journal. 2006; 172:.422-431.
- Engelhardt B, Ransohoff RM. Capture, crawl, cross: The T cell code to breach the blood brain barriers. Trends in immunology. 2012; 33: 579-589.
- Hladky SB, Barrand MA. Fluid and ion transfer across the blood–brain and blood–cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids and Barriers of the CNS. 2016; 13: 19. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27799072
- Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. The Journal of clinical investigation. 2013; 123: 1299-1309.
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Science translational medicine. 2012; 4: 147ra111-147ra111.
- Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochemical research. 2015; 40: 2583-2599.
- Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal fluid research. 2004; 1: 2.
- Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal fluid research. 2005; 2:.6.
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, et al. Impairment of paravascular clearance pathways in the aging brain. Annals of neurology. 2014; 76: 845-861.
- Kulik T, Kusano Y, Aronhime S, Sandler AL, Winn HR. Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacology. 2008; 55: 281-288.
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010; 58: 1094-1103.
- Murtha LA, Yang Q, Parsons MW, Levi CR, Beard DJ, et al. Cerebrospinal fluid is drained primarily via the spinal canal and olfactory route in young and aged spontaneously hypertensive rats. Fluids and Barriers of the CNS. 2014; 11: 12.
- Nedergaard M. Garbage truck of the brain. Science. 2013; 340: 1529-1530.
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nature Reviews Neuroscience. 2011; 12: 169.
- Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004; 54: 131-142.
- Pizzo ME, Thorne RG. The extracellular and perivascular spaces of the brain. In Brain edema Academic Press. 2017; 105-127.
- Prince EA, Ahn SH. Basic vascular neuroanatomy of the brain and spine: what the general interventional radiologist needs to know. In Seminars in interventional radiology. Thieme Medical Publishers. 2013; 30: 234-239.
- Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain research. 1985; 326: 47-63. PubMEd: https://www.ncbi.nlm.nih.gov/pubmed/3971148
- Rennels ML, Blaumanis OR, Grady PA. Rapid solute transport throughout the brain via paravascular fluid pathways. Advances in neurology. 1990; 52: 431-439.
- Syková E, Nicholson C. Diffusion in brain extracellular space. Physiological reviews. 2008; 88: 1277-1340.
- Tait MJ, Saadoun S, Bell BA, Papadopoulos MC. Water movements in the brain: role of aquaporins. Trends in neurosciences. 2008; 31: 37-43. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18054802
- Thorne RG, Nicholson C. in vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proceedings of the National Academy of Sciences. 2006; 103: 5567-5572.
- Thorne RG. Primer on central nervous system structure/function and the vasculature, ventricular system, and fluids of the brain. Drug Delivery to the Brain. Springer. 2014; 685-706.
- Thrane AS, Thrane VR, Nedergaard M. Drowning stars: reassessing the role of astrocytes in brain edema. Trends in neurosciences. 2014; 37: 620-628.
- Thrane VR, Thrane AS, Plog BA, Thiyagarajan M, Iliff JJ, et al. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Scientific reports. 2013; 3: 2582.
- Trevaskis NL, Kaminskas LM, Porter CJ. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nature Reviews Drug Discovery. 2015; 14: 781-803.
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013; 342: 373-377.
- Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. Journal of anatomy. 1990; 170: 111.
- Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015; 163: 1064-1078. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26590417
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nature Reviews Neuroscience. 2011; 12: 723.
- Luisetto M, Ahmadabadi BN, Rafa AY, Sahu RK, Cabianca L, et al. The turing machine theory for some spinal cord and brain condition, A toxicological - antidotic depurative approach. J Neurosci Neurol Disord. 2019; 3: 102-134.
- Luisetto Mauro, Ibrahim G, Oleg Latyschev, et al. The Evolution of the Nervous System as Model for Search New Pharmacological Strategies in Human Neurological Condition. American Journal of Biomedical Science & Research. 2019; 5.
- Muhammad Shehzad Khan. Glymphatic clearance of simulated silicon dispersion in mouse brain analyzed by laser induced breakdown spectroscopy. Helion. 2020; 6.