More Information
Submitted: November 17, 2022 | Approved: November 22, 2022 | Published: November 23, 2022
How to cite this article: Patel S, Wheeler SE, Anderson A, Pinto LM, Shurin MR. SARS-CoV-2 antibody response to third dose vaccination in a healthy cohort. Insights Clin Cell Immunol. 2022; 6: 008-013.
DOI: 10.29328/journal.icci.1001020
Copyright License: © 2022 Patel S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: SARS-CoV-2; Boosting vaccination; Antibody; Serology; Vaccine response
SARS-CoV-2 antibody response to third dose vaccination in a healthy cohort
Simmi Patel1*, Sarah E Wheeler1,2, Adam Anderson3, Lisa Pinto3 and Michael R Shurin1,2,4
1Department of Pathology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
2Department of Pathology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
3Bio-Rad Laboratories, Inc., Benicia, California, USA
4Department of Immunology, University of Pittsburgh Medical Center and University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
*Address for Correspondence: Simmi Patel, MD, Department of Pathology, University of Pittsburgh Medical Center, 3477 Euler Way, CLB Room 4024, Pittsburgh, PA 15213, USA, Email: [email protected]
Severe acute respiratory syndrome coronavirus 2, also known as SARS-CoV-2 is the main cause of the coronavirus pandemic which began in 2019. In the last few years, vaccines have been shown to be effective against SARS-CoV-2 infection [1,2]. Production of antibodies starts within days to weeks after the first dose and studies have established the need for a second dose for improved efficacy [3,4]. Recent studies suggest that humoral immunity against SARS-CoV-2 associated with natural infection or vaccination may persist for several months however anti-viral antibodies will wane over time [5]. There are four major immunogenic proteins in SARS-CoV-2: spike, envelope, membrane and nucleocapsid which are encoded by their respective genes, S, E, M and N genes [6,7]. The S protein contains two subunits - S1 and S2, which mediate receptor binding and membrane fusion. Viral particles enter the host through a virus-host cell membrane fusion which requires a conformational change of the S protein. The receptor binding domain (RBD) (found on S1) binds to ACE2 (angiotensin-converting enzyme 2) on the host cell. This allows for a necessary conformation change of the S2 subunit permitting the membrane fusion [8,9]. Both natural infection of SARS-CoV-2 and vaccination allow for production and detection of anti-S1, S2, and RBD antibodies. After the second vaccine dose for most healthy volunteers, high levels of IgM, IgG anti-SARS-Cov-2 spike proteins S and RBD can be detected in circulation providing a surrogate measure of mRNA-vaccine induced immunity [10,11]. Natural infection is measured through anti-nucleocapsid antibodies which continues to serve as a marker for SARS-CoV-2 infection and it is not induced by spike protein-encoding vaccines [3].
The mRNA vaccine series for SARS-CoV-2 comprises of two doses for both Pfizer-BioNTech (30 µg, 0.3 mL each with three weeks apart) and Moderna (100 µg of 0.5 mL with one month apart) [12,13]. Initial doses of the vaccine showed elevated RBD IgG antibodies with steady titers at approximately three weeks with a significant increase after approximately seven days. After the second dose, levels of antibodies to the spike protein were increased at approximately five weeks, compared to that of 2 weeks - 3 weeks [14]. Similarly, other studies reported that amongst previously uninfected healthy people, anti-spike protein titers after one dose of the vaccine were comparable to the peak of the anti-spike protein titers seen in those with natural infection without prior vaccination [15]. Additional studies also show agreement with results of an antibody response in the first vaccine dose in individuals with preexisting immunity which is equal to or even exceeds titers in naïve individuals after second vaccine doses [3,12].
There have been numerous attempts to standardize the quantification of the level of antibodies, however with variations in manufacturers, it is difficult to create such a standardized vaccine correlate of protection [16]. The goal of this study was to determine the change in the level of antibodies to RBD, S1, S2 and nucleocapsid SARS-CoV-2 antigens in healthy volunteers receiving their third dose of SARS-CoV-2 mRNA vaccines at two and four weeks following third dose.
Study design and participants
Eligible participants were healthy individuals 18 years or older. There were a total of 31 participants who received two injections of one of the United States (US) emergency use authorized (EUA) mRNA vaccines (Pfizer/BioNTech or Moderna) and who would receive their third dose during the study. There were no enrolled participants who were subsequently excluded. The key exclusion criteria included: unwell at the time of recruitment, immunosuppression from disease or treatment, anemia, treatment for anemia or iron deficiency and known pregnancy. Written informed consent was obtained from each participant before enrollment in this study. Written approval of the study protocol and informed consent were obtained from the relevant independent ethics committee/Institutional Review Board (IRB) of the University of Pittsburgh (study #:21090015) and all work was performed in accordance with the Declaration of Helsinki. Blood samples were collected within the week prior to the third vaccine dose, two weeks after third dose and then four weeks after third dose. Length of time between second and third vaccine doses was a median 281 days (range: 200 days - 328 days).
Detection of virus-specific antibodies
Blood samples were collected into serum gel separator tubes and centrifuged after complete clotting at room temperature. Serum was collected from participants at the relevant time points and stored at -30 °C for up to four months before analysis. SARS-CoV-2 antibody assays were performed in the CLIA certified high-complexity clinical laboratories at the University of Pittsburgh Medical Center. We used the BioPlex 2200 CoV-2 IgG Panel (Bio-Rad laboratories, Inc., Hercules, CA) which is a multiplex assay for qualitative (IgG screening) and semi-quantitative (U/mL) detection of IgG class antibodies against RBD, S1, S2 and nucleocapsid proteins of SARS-CoV-2 virus in human serum and plasma. This assay is for research use only in the US but was run in accordance with all local clinical laboratory requirements. The analytical measuring range for the semi-quantitative assay is 1 to 100 U/mL with a threshold of ≥ 10 U/mL being positive. There are onboard dilutions of 1:8, 1:16 and 1:32. Samples with levels higher than 3200 U/mL were manually diluted at 1:10 with the assay dilution buffer. Performance studies showed a specificity of 99.8% and overall sensitivity to 96.3% according to the manufacturer’s instructions.
Statistical analysis
Data was analyzed using non-parametric t tests by Prism (version 9; GraphPad, San Diego, CA) for all analysis and data presentation. We considered p values of < 0.05 to be significant. The data are presented as means ± the standard errors of the mean (SEM).
A total of 31 healthy volunteers were included (n = 31). Of those, 29 were health care workers. 45% of these participants were males, 55% were females. Five out of 31 identified as Asian ethnically and 26 out of 31 identified as Caucasian. 25% of subjects received Pfizer mRNA vaccine and 75% received Moderna (Table 1). All participants received the same brand of vaccine for all three doses.
| Table 1: Demographic information. | |
| Demographic Information | Number (%) |
| Gender | |
| Male | 14 (45%) |
| Female | 17 (55%) |
| Race | |
| Asian | 5 (16%) |
| Caucasian | 26 (84%) |
| Pre-booster vaccine | |
| Pfizer | 8 (25%) |
| Moderna | 23 (75%) |
| Vaccine type | |
| Pfizer | 8 (25%) |
| Moderna | 23 (75%) |
| Use of immunosuppressive drugs | 0 (0%) |
| COVID-19 prior to booster | 1 (3%) |
| Known COVID-19 exposure prior to booster | 2 (6%) |
| Use of post-exposure prophylaxis monoclonal antibody therapy | 0 (0%) |
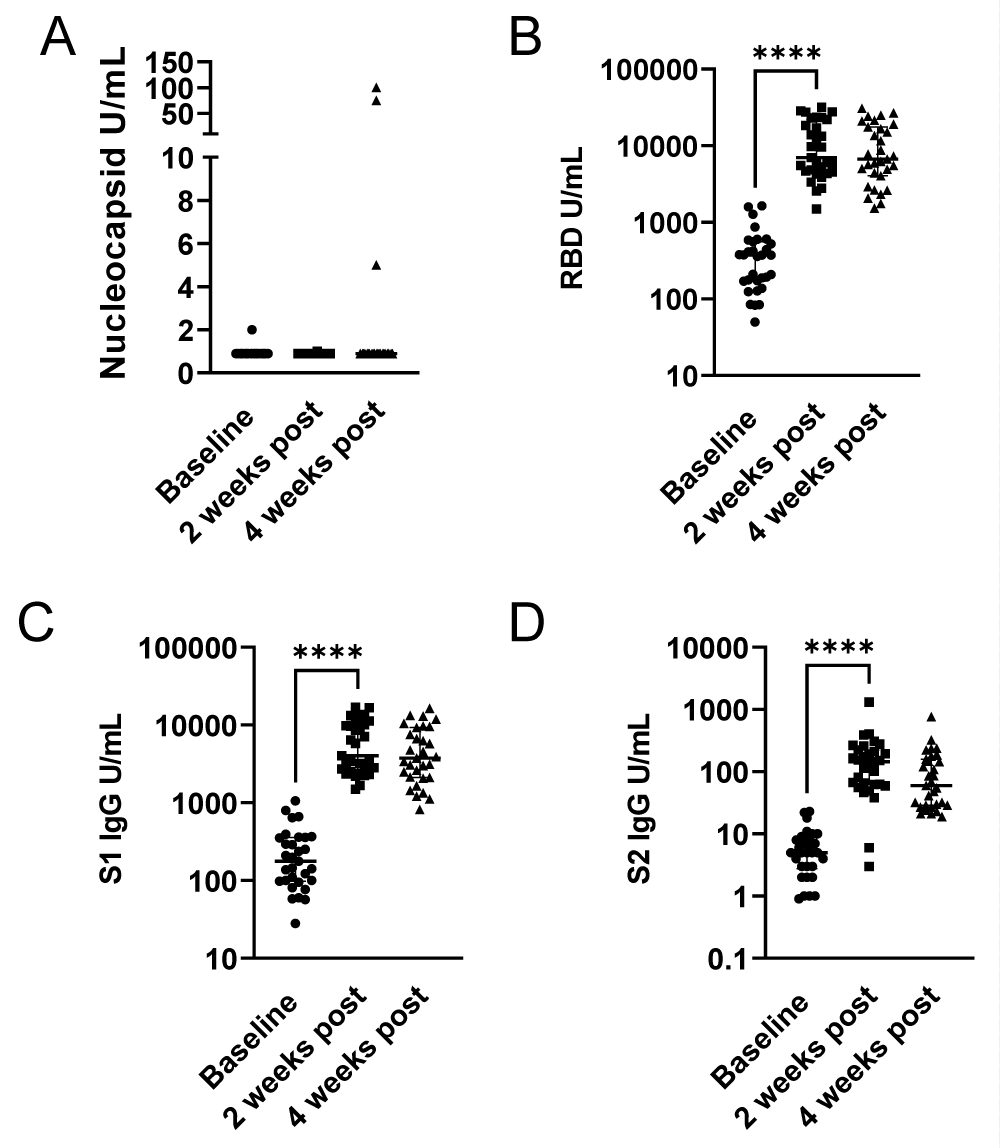
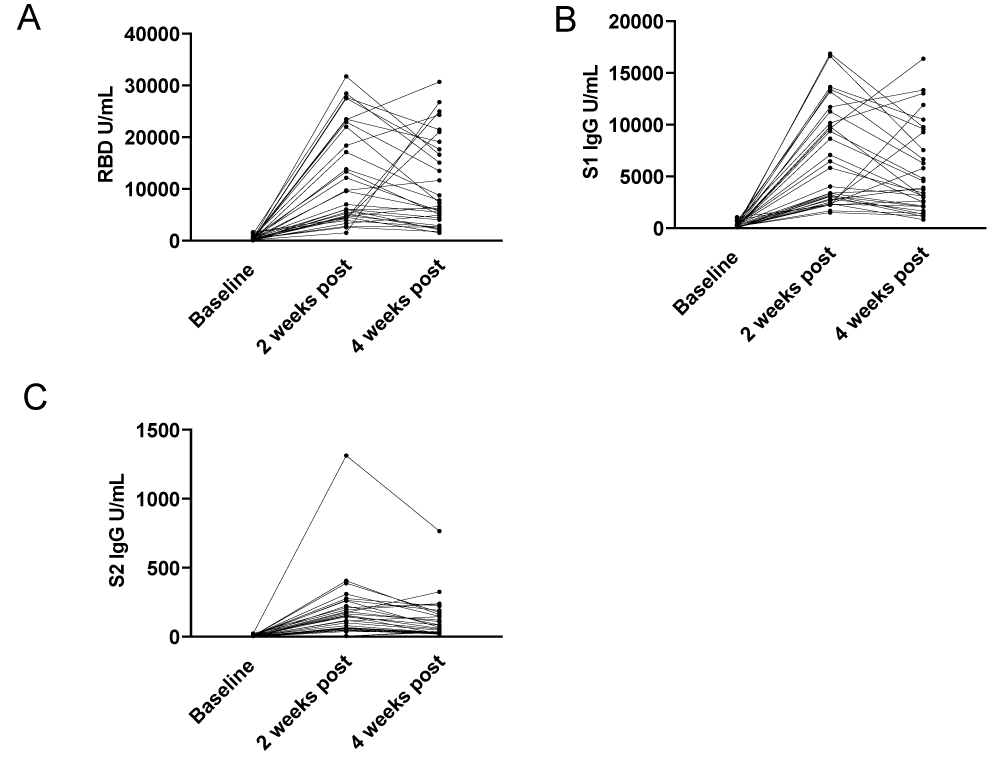
To assess the change in SARS-CoV-2 IgG following third vaccine dosing, we looked at antibody responses to nucleocapsid to control for possible confounding infection with SARS-CoV-2, and spike 1 (S1), spike 2 (S2) and the Receptor Binding Domain (RBD). After third dose vaccination, antibody levels for RBD, S1, and S2 were significantly increased at 2 weeks and overall remained elevated at 4 weeks (Figure 1A-D). Within this cohort we detected two participants who had an increase in nucleocapsid IgG levels between weeks 2 and 4 following their third vaccine dose, indicative of likely SARS-CoV-2 exposure following their third vaccine dose (Figure 1A). With the exclusion of these two participants, p - value remained the same (p = 0.2500) and was not significant. For both RBD and S1, the baseline serology had detectable antibodies and there was a significant increase 2 weeks post third dose (p < 0.0001) from baseline. For anti-RBD and S1 antibodies, between 2- and 4-weeks post third dose, titers were declining however aggregate analysis did not demonstrate a significant difference (p varied from 0.0502 - 0.0759) (Figures 1B,1C). For S2, most participants were negative for S2 antibodies at baseline. There was a significant increase between baseline and 2 weeks post third dose (p < 0.0001) with a significant waning of detectable antibodies by 4 weeks post third dose (p < 0.0002 week 2 compared to week 4). However, variation in the detection of S2 could be due to either an assay-specific or a physiologic response (Figure 1D). We noted that although there is no significant difference in aggregate analysis between 2 and 4 weeks for RBD/S1, for several participants there is a significant decrease in detectable IgG, while for others there is a delayed increase (Figure 2A-C). Particularly, values for RBD, S1 and S1 IgG antibodies did not fall below 1500 U/mL, 9650 U/mL and 175 U/mL respectively at 2 weeks post third dose, or 1530 U/mL, 820 U/mL and 55 U/mL respectively at 4 weeks post third dose demonstrating a persistence vaccine-induced antibody response.
Figure 1: Antibody responses to third dose of mRNA vaccines. Blood specimens were collected from the cohort within one week before (0 weeks) and after the third dose of mRNA vaccines at two and four weeks. U/mL of binding antibodies are presented for each participant for (A) nucleocapsid IgG, (B) receptor binding domain IgG, (C) spike 1 subdomain IgG and (D) spike 2 subdomain IgG. Specimens are reactive at > 10 U/mL. Comparisons were performed by Wilcoxon and Mann-Whitney Rank Sum test, ****, p - value < 0.001.
Figure 2: Temporal responses to third dose of mRNA vaccines. Blood specimens were collected from the cohort within one week before (0 weeks) and after the third dose of mRNA vaccines at two and four weeks. U/mL of binding antibodies are presented for each participant for (A) receptor binding domain IgG, (B) spike 1 subdomain IgG and (C) spike 2 subdomain IgG. Specimens are reactive at > 10 U/mL. Lines connect individual participants.
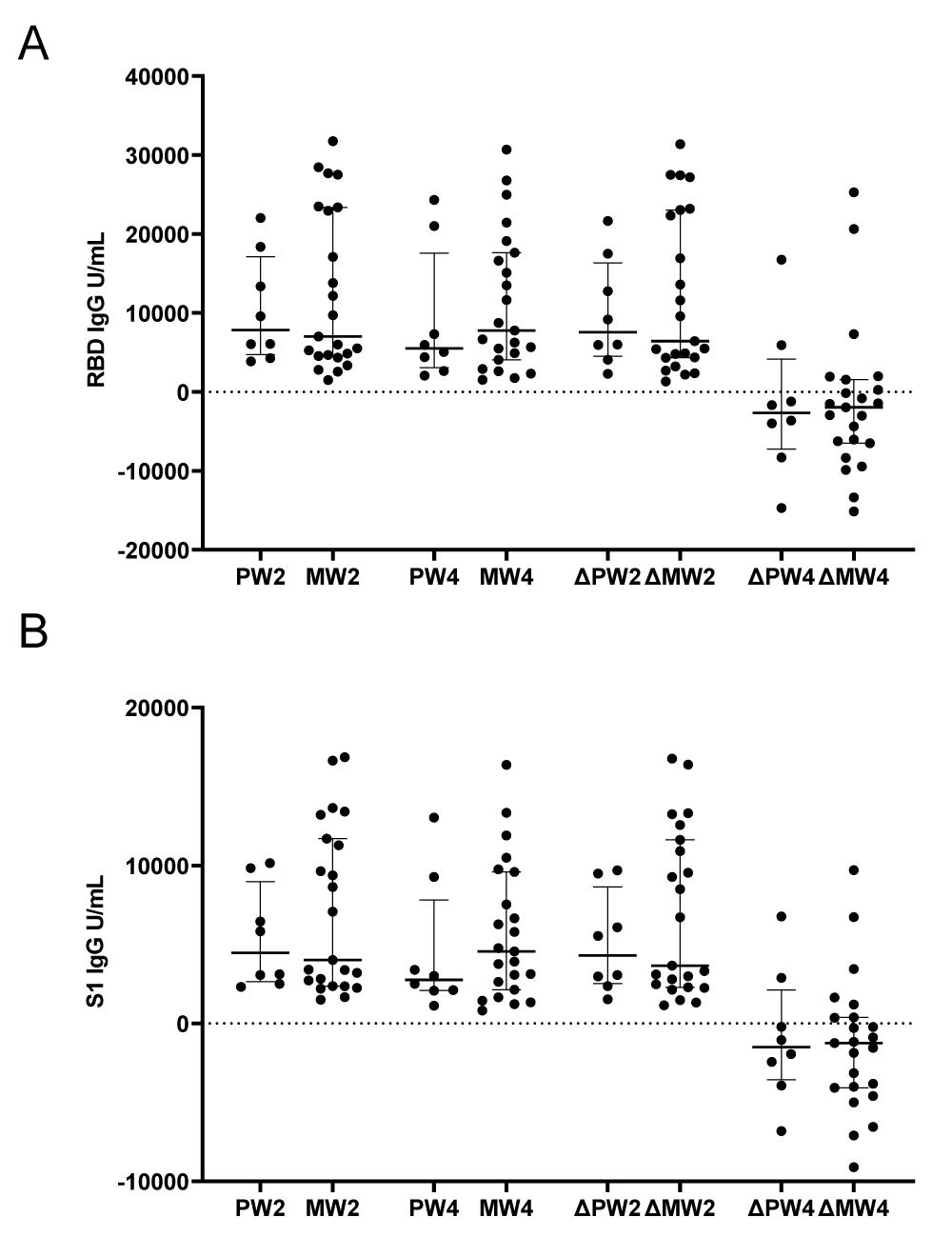
One of the goals in this study was to assess possible variation between the two mRNA vaccines. We assessed RBD and S1 antibody levels and found no significant difference between the two vaccine types at 2 weeks and 4 weeks post third vaccine dose (Figure 3A,B). We calculated the difference between baseline and 2 weeks, as well as the difference between 2 and 4 weeks post third dose with both vaccine types to include both RBD and S1 antibodies and still found no difference between the antibody response to third dose vaccination. The p - value for RBD ranged from 0.5496 to 0.9824 and for S1 ranged from 0.3620 to 0.8078 (Figure 3A,B).
Figure 3: Comparison of Pfizer and Moderna antibody responses. Blood specimens were collected from the cohort within one week before (0 weeks) and after the third dose of mRNA vaccines at two and four weeks. U/mL of binding antibodies are presented for each participant for (A) receptor binding domain IgG, (B) spike 1 subdomain IgG. Abbreviations: Pfizer week 2 (PW2), Moderna week 2 (MW2), Pfizer week 4 (PW4), Moderna week 4 (MW4), change (Δ) for Pfizer (P) or Moderna (M) between weeks 2 and 0 (W2) or between weeks 4 and 2 (W4). Horizontal dashed line indicates positivity threshold (10 U/mL).
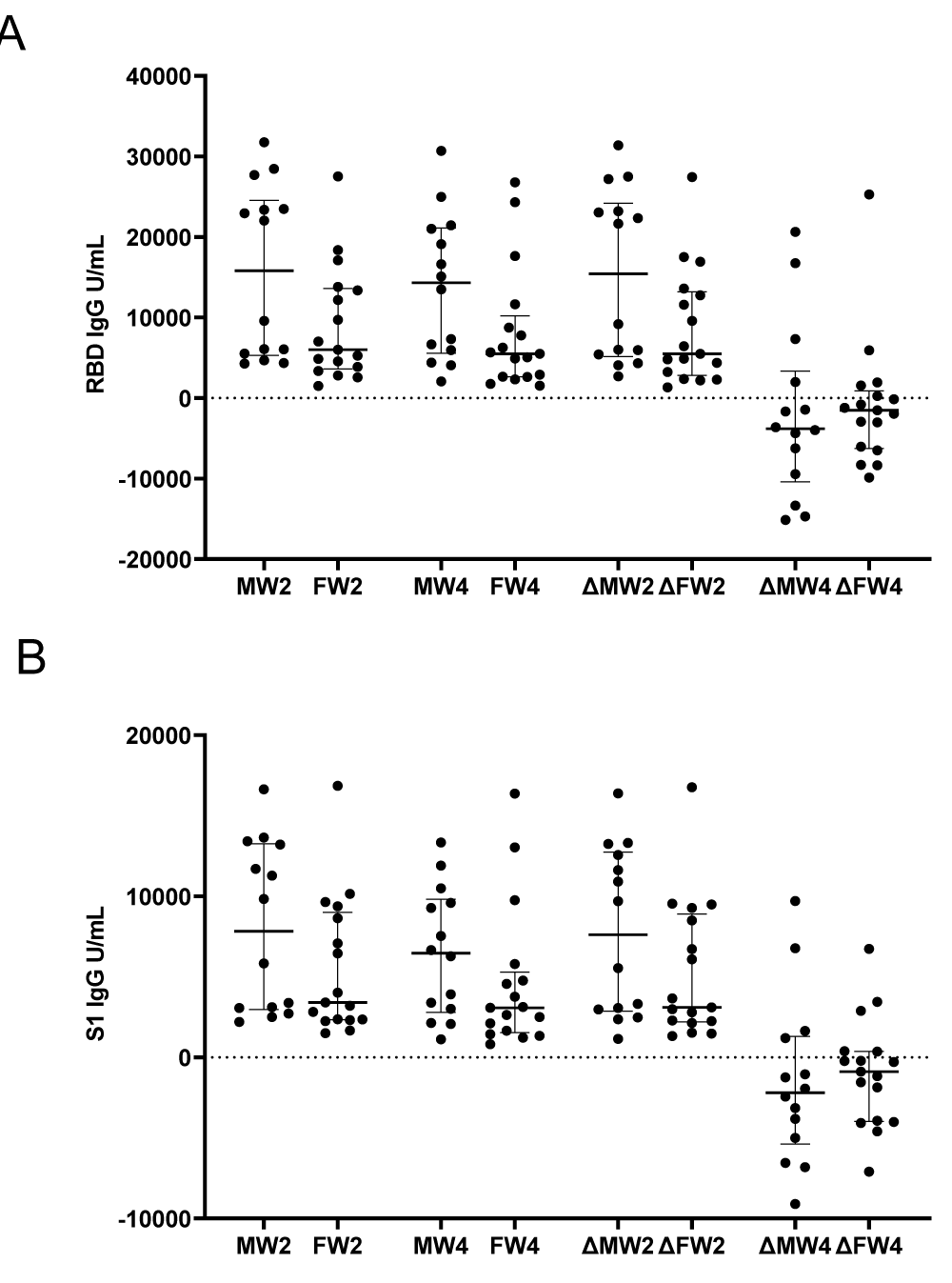
Lastly, we considered if there may be a difference between genders for both vaccines. We found no significant difference between males and females amongst vaccine types assessing RBD and S1 antibodies. P - value ranged from: RBD 0.0586 to 0.4683 and S1 ranged from: 0.1278 to 0.4683 (Figure 4A,B).
Figure 4: Comparison of male and female antibody responses. Blood specimens were collected from the cohort within one week before (0 weeks) and after the third dose of mRNA vaccines at two and four weeks. U/mL of binding antibodies are presented for each participant for (A) receptor binding domain IgG, (B) spike 1 subdomain IgG. Abbreviations: Male week 2 (MW2), Female week 2 (FW2), Male week 4 (MW4), Female week 4 (FW4), change (Δ) for Male (M) or Female (F) between weeks 2 and 0 (W2) or between weeks 4 and 2 (W4). Horizontal dashed line indicates positivity threshold (10 U/mL).
Over the last two years, numerous SARS-Co-V-2 variants have emerged and were classified as WHO variants of concern. Our study looked at the serological response of nucleocapsid, RBD, S1 and S2 antibodies after a booster vaccination of healthy donors. Specifically, we looked at the antibody response at two and four weeks post third doses. We found there was a vaccine-induced response in RBD, S1 and S2 IgG antibody levels by both vaccines, however there was no statistically significant difference between the Moderna and Pfizer vaccines. Similar studies also show an increase in antibody levels after third doses utilizing different assays [16,17].
After the third dose, we found, as expected, a significant increase in antibody levels for RBD, S1 and S2 (p < 0.0001 for two weeks post third dose and p - value range from: 0.0002 to 0.0759 for four weeks post third dose). Our observations are concurrent with other studies assessing antibody levels post third doses; several authors observed an antibody peak with values of antibody levels 2 to 3 times higher than baseline values. Interestingly, in our study, at 28 days after the third dose, there was a trend of decreasing antibody levels which was most prominent in S2 IgG antibody levels (minimum level of S2 antibody reported: 55 U/mL). Several other studies report similar findings in which while anti-S2 antibodies cross neutralize viral SARS-CoV-2 particles, they appear to decline earlier than anti-RBD antibodies thus decreasing its potency [18,19].
Our prior work using this assay during the primary vaccination series demonstrated average values of 5490 U/mL S1 and 15,300 U/mL RBD following second vaccination. Semiquantitative levels of the response from both doses are similar to the levels identified post third vaccination in our cohort for this specific study [3]. Similarly, there were no statistically significant differences in the potency of immune response to RBD protein after first and second doses of either vaccine.
The small sample size limits broad generalizations but provides a starting point for further work. Multicenter studies are needed to assess long-term vaccination plans as SARS-CoV-2 continues to change globally. Incorporation of nucleocapsid information allowed us to incorporate natural infection as an exclusion criterion in analyses, however, the exclusion of participants with nucleocapsid antibody positivity did not change the significance of the associated p - values. Additionally, the follow-up time of our study was relatively short. Timely reporting for follow-up data is important in order to adjust immunization strategies in the context of the pandemic as variants emerge.
In conclusion, our serological data on third dose vaccine-induced antibodies, controlling for SARS-CoV-2 exposure through detection of anti-nucleocapsid antibodies, finds significant increases in antibody response to both mRNA vaccines with no significant difference between the vaccines. Numerous studies indicate the need for multicomponent vaccines which include and are not limited to the S protein as new variants may escape immune targeting [20-24]. Immunity progressively wanes through the course of two to four weeks for many participants implying that use of serology for epidemiologic studies or to assess vaccine response may need to consider time from vaccination or infection.
Author contributions
Concept and design: Michael Shurin, Sarah Wheeler, Simmi Patel.
Administrative support: Adam Anderson.
Provision and study materials or patients: Adam Anderson, Lisa Pinto, Sarah Wheeler, Michael Shurin and Simmi Patel.
Collection and assembly of data: Michael Shurin and Simmi Patel.
Data analysis and interpretation: Sarah Wheeler.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
We thank Mary Yost, Vincent Maskivish and Maria Desantis for help with blood collection and preparation of serum samples and we thank Bio-Rad Laboratories for providing key reagents and supporting antibody detection. We also thank all volunteers participating in the study. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
We confirm that all relevant ethical guidelines have been followed, and all necessary IRB and/or ethics committee approvals have been obtained. Bio-Rad Laboratories had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec 31;383(27):2603-2615. doi: 10.1056/NEJMoa2034577. Epub 2020 Dec 10. PMID: 33301246; PMCID: PMC7745181.
- Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Gruber WC, Jansen KU; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021 Nov 4;385(19):1761-1773. doi: 10.1056/NEJMoa2110345. Epub 2021 Sep 15. PMID: 34525277; PMCID: PMC8461570.
- Wheeler SE, Shurin GV, Yost M, Anderson A, Pinto L, Wells A, Shurin MR. Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects. Microbiol Spectr. 2021 Sep 3;9(1):e0034121. doi: 10.1128/Spectrum.00341-21. Epub 2021 Aug 4. PMID: 34346750; PMCID: PMC8552678.
- Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, Al Khatib HA, Coyle P, Ayoub HH, Al Kanaani Z, Al Kuwari E, Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Abdul Rahim HF, Nasrallah GK, Al Kuwari MG, Al Romaihi HE, Butt AA, Al-Thani MH, Al Khal A, Bertollini R, Abu-Raddad LJ. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N Engl J Med. 2021 Dec 9;385(24):e83. doi: 10.1056/NEJMoa2114114. Epub 2021 Oct 6. PMID: 34614327; PMCID: PMC8522799.
- Flisiak R, Pawłowska M, Rogalska-Płońska M, Bociąga-Jasik M, Kłos K, Piekarska A, Zarębska-Michaluk D. Effect of COVID-19 on Anti-S Antibody Response in Healthcare Workers Six Months Post-Vaccination. Vaccines (Basel). 2021 Nov 15;9(11):1325. doi: 10.3390/vaccines9111325. PMID: 34835257; PMCID: PMC8618383.
- Bal A, Pozzetto B, Trabaud MA, Escuret V, Rabilloud M, Langlois-Jacques C, Paul A, Guibert N, D'Aubarède-Frieh C, Massardier-Pilonchery A, Fabien N, Goncalves D, Boibieux A, Morfin-Sherpa F, Pitiot V, Gueyffier F, Lina B, Fassier JB, Trouillet-Assant S; COVID SER Study Group. Evaluation of High-Throughput SARS-CoV-2 Serological Assays in a Longitudinal Cohort of Patients with Mild COVID-19: Clinical Sensitivity, Specificity, and Association with Virus Neutralization Test. Clin Chem. 2021 Apr 29;67(5):742-752. doi: 10.1093/clinchem/hvaa336. PMID: 33399823; PMCID: PMC7929008.
- Xia X. Domains and Functions of Spike Protein in Sars-Cov-2 in the Context of Vaccine Design. Viruses. 2021 Jan 14;13(1):109. doi: 10.3390/v13010109. PMID: 33466921; PMCID: PMC7829931.
- Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020 Sep;41(9):1141-1149. doi: 10.1038/s41401-020-0485-4. Epub 2020 Aug 3. PMID: 32747721; PMCID: PMC7396720.
- Bonifacius A, Tischer-Zimmermann S, Dragon AC, Gussarow D, Vogel A, Krettek U, Gödecke N, Yilmaz M, Kraft ARM, Hoeper MM, Pink I, Schmidt JJ, Li Y, Welte T, Maecker-Kolhoff B, Martens J, Berger MM, Lobenwein C, Stankov MV, Cornberg M, David S, Behrens GMN, Witzke O, Blasczyk R, Eiz-Vesper B. COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity. 2021 Feb 9;54(2):340-354.e6. doi: 10.1016/j.immuni.2021.01.008. PMID: 33567252; PMCID: PMC7871825.
- Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, Schaefer-Babajew D, Cipolla M, Gaebler C, Lieberman JA, Oliveira TY, Yang Z, Abernathy ME, Huey-Tubman KE, Hurley A, Turroja M, West KA, Gordon K, Millard KG, Ramos V, Da Silva J, Xu J, Colbert RA, Patel R, Dizon J, Unson-O'Brien C, Shimeliovich I, Gazumyan A, Caskey M, Bjorkman PJ, Casellas R, Hatziioannou T, Bieniasz PD, Nussenzweig MC. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021 Apr;592(7855):616-622. doi: 10.1038/s41586-021-03324-6. Epub 2021 Feb 10. PMID: 33567448; PMCID: PMC8503938.
- Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Şahin U, Gruber WC. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020 Dec 17;383(25):2439-2450. doi: 10.1056/NEJMoa2027906. Epub 2020 Oct 14. PMID: 33053279; PMCID: PMC7583697.
- Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, Bermúdez-González MC, Bielak DA, Carreño JM, Chernet RL, Eaker LQ, Ferreri ED, Floda DL, Gleason CR, Hamburger JZ, Jiang K, Kleiner G, Jurczyszak D, Matthews JC, Mendez WA, Nabeel I, Mulder LCF, Raskin AJ, Russo KT, Salimbangon AT, Saksena M, Shin AS, Singh G, Sominsky LA, Stadlbauer D, Wajnberg A, Simon V. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021 Apr 8;384(14):1372-1374. doi: 10.1056/NEJMc2101667. Epub 2021 Mar 10. PMID: 33691060; PMCID: PMC8008743.
- Amanat F, Thapa M, Lei T, Sayed Ahmed SM, Adelsberg DC, Carreno JM, et al. The plasmablast response to SARS-CoV-2 mRNA vaccination is dominated by non-neutralizing antibodies and targets both the NTD and the RBD. medRxiv. 2021; 2021.03.07.21253098.
- Yoshimura Y, Sasaki H, Miyata N, Miyazaki K, Tachikawa N. Antibody response after COVID-19 vaccine BNT162b2 on health care workers in Japan. J Infect Chemother. 2021 Dec;27(12):1713-1715. doi: 10.1016/j.jiac.2021.08.008. Epub 2021 Aug 12. PMID: 34412983; PMCID: PMC8358099.
- Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, Noursadeghi M, Boyton RJ, Semper A, Moon JC. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021 Mar 20;397(10279):1057-1058. doi: 10.1016/S0140-6736(21)00501-8. Epub 2021 Feb 25. PMID: 33640038; PMCID: PMC7972310.
- Vietri MT, D'Elia G, Caliendo G, Passariello L, Albanese L, Molinari AM, Angelillo IF. Antibody levels after BNT162b2 vaccine booster and SARS-CoV-2 Omicron infection. Vaccine. 2022 Sep 16;40(39):5726-5731. doi: 10.1016/j.vaccine.2022.08.045. Epub 2022 Aug 26. PMID: 36041940; PMCID: PMC9411148.
- Vietri MT, Albanese L, Passariello L, D'Elia G, Caliendo G, Molinari AM, Angelillo IF. Evaluation of neutralizing antibodies after vaccine BNT162b2: Preliminary data. J Clin Virol. 2022 Jan;146:105057. doi: 10.1016/j.jcv.2021.105057. Epub 2021 Dec 14. PMID: 34923323; PMCID: PMC8670104.
- Lanz TV, Brewer RC, Jahanbani S, Robinson WH. Limited Neutralization of Omicron by Antibodies from the BNT162b2 Vaccination against SARS-CoV-2. 2022; https://www.researchsquare.com/article/rs-1518378/v1
- Matula Z, Gönczi M, Bekő G, Kádár B, Ajzner É, Uher F, Vályi-Nagy I. Antibody and T Cell Responses against SARS-CoV-2 Elicited by the Third Dose of BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) Vaccines Using a Homologous or Heterologous Booster Vaccination Strategy. Vaccines (Basel). 2022 Mar 30;10(4):539. doi: 10.3390/vaccines10040539. PMID: 35455288; PMCID: PMC9025723.
- Cerutti G, Guo Y, Zhou T, Gorman J, Lee M, Rapp M, Reddem ER, Yu J, Bahna F, Bimela J, Huang Y, Katsamba PS, Liu L, Nair MS, Rawi R, Olia AS, Wang P, Zhang B, Chuang GY, Ho DD, Sheng Z, Kwong PD, Shapiro L. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe. 2021 May 12;29(5):819-833.e7. doi: 10.1016/j.chom.2021.03.005. Epub 2021 Mar 12. PMID: 33789084; PMCID: PMC7953435.
- Suthar MS, Zimmerman MG, Kauffman RC, Mantus G, Linderman SL, Hudson WH, Vanderheiden A, Nyhoff L, Davis CW, Adekunle O, Affer M, Sherman M, Reynolds S, Verkerke HP, Alter DN, Guarner J, Bryksin J, Horwath MC, Arthur CM, Saakadze N, Smith GH, Edupuganti S, Scherer EM, Hellmeister K, Cheng A, Morales JA, Neish AS, Stowell SR, Frank F, Ortlund E, Anderson EJ, Menachery VD, Rouphael N, Mehta AK, Stephens DS, Ahmed R, Roback JD, Wrammert J. Rapid Generation of Neutralizing Antibody Responses in COVID-19 Patients. Cell Rep Med. 2020 Jun 23;1(3):100040. doi: 10.1016/j.xcrm.2020.100040. Epub 2020 Jun 8. PMID: 32835303; PMCID: PMC7276302.
- Huhn G, Poorbaugh J, Zhang L, Beasley S, Nirula A, Brothers J, Welbel S, Wilson J, Gillani S, Weber KM, Morack R, Keckler K, Benschop RJ. COVID-19 symptom relationship to antibody response and ACE2 neutralization in recovered health systems employees before and after mRNA BNT162b2 COVID-19 vaccine. PLoS One. 2022 Sep 9;17(9):e0273323. doi: 10.1371/journal.pone.0273323. PMID: 36083883; PMCID: PMC9462709.
- Cassone A, Cauda R. Multicomponent vaccines to fight SARS-CoV-2 variants of concern. Vaccine. 2021 Nov 26;39(48):6969-6971. doi: 10.1016/j.vaccine.2021.10.069. Epub 2021 Nov 1. PMID: 34743927; PMCID: PMC8557988.
- Poland GA, Ovsyannikova IG, Kennedy RB. The need for broadly protective COVID-19 vaccines: Beyond S-only approaches. Vaccine. 2021 Jul 13;39(31):4239-4241. doi: 10.1016/j.vaccine.2021.06.028. Epub 2021 Jun 14. PMID: 34167836; PMCID: PMC8200305.