Research Article
Association of Toll-like receptor 2, 4, and 9 gene polymorphism with high altitude induced thrombosis patients in Indian population

Swati Sharma1#, Iti Garg1*#, Gauri Mishra2, Babita Kumari1, Lilly Ganju1 and Bhuvnesh Kumar1
1Defence Institute of Physiology & Allied Sciences, Timarpur, Delhi, India
2Amity University, Noida, India
#Equal first authorship
*Address for Correspondence: Iti Garg, Genomics Division, Defence Institute of Physiology and Allied Sciences, Lucknow Road, Timarpur, Delhi, India, Tel: 91-11-23883190; Fax: 91-11-23914790; Email: [email protected]
Dates: Submitted: 10 January 2019; Approved: 07 February 2019; Published: 08 February 2019
How to cite this article: Sharma S, Garg I, Mishra G, Kumari B, Ganju L, et al. Association of Toll-like receptor 2, 4, and 9 gene polymorphism with high altitude induced thrombosis patients in Indian population. Insights Clin Cell Immunol. 2019; 3: 006-015. DOI: 10.29328/journal.icci.1001008
Copyright License: © 2019 Sharma S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: VTE; TLRs; SNP; Genotyping
Abstract
Venous Thromboembolism (VTE) is a multifactorial disease that is influenced by individual genetic background and various environmental factors, high altitude (HA) being the one. HA exposure may cause release of several damage associated molecular patterns (DAMPs), which act as ligand for various immune receptors. Previous studies on western population involving SNPs analysis of TLRs demonstrated that TLRs are involved in development and progression of several cardiovascular diseases. But, no such study has been done in Indian population in context of HA exposure. TLRs, being receptors play a significant role in manifestation and elimination of diseases by recognition of specific ligands and downstream signal transduction therefore; the genetic variation in TLRs could be implicated for imparting varying response of individuals to discrete diseases.
Therefore, in accordance with it, in present study changes in protein structures of TLR2 and TLR4 due to presence of SNP were accessed by in-silico tools to observe whether the mutation has effect on protein structure and integrity which further influencing its function. The results showed that SNP harbouring protein has decreased functional pockets, thus may be protective for disease. Taking this lead further to genotypic level, first time association between Toll-like receptor genes polymorphism and risk of high altitude induced venous thrombosis is analyzed in Indian population by PCR RFLP method. Though the result showed initial trend that TLR2 and TLR9 SNP are monomrphic in distribution and for TLR4 there was no significant difference in distribution of SNP between healthy and HA-DVT group, these SNPs have potential to be used as susceptibility markers if studied in large population size.
Graphical Abstract
Introduction
Venous thromboembolism (VTE) is characterised as one of the widespread multifactorial disease, which is associated with several inherited and acquired predisposing factors [1-3]. Hypoxia, an unique environment condition at high altitudes is being characterised as one of the acquired risk factor for VTE as it predisposes otherwise healthy individuals to venous thrombosis. There are various reports giving insight about the incidences of deep vein thrombosis (DVT) and pulmonary embolism [4,5], portal vein thrombosis [7] and cerebral venous thrombosis [7] at high altitude [8]. Anand et al., has reported that long term stay at HA is associated with 30% increase in risk of vascular thrombosis [9]. Still the underlying molecular mechanism of hypoxia-induced venous thrombosis is not well established. The annual incidence of occurrence of VTE globally is 6 to 29 persons per lakh in distinct populations [10]. Whereas, In Indian population very few incidences have been reported mainly because of the lack of epidemiological data for VTE [11]. The inherited risk factors affecting VTE includes mutations in genes involved in coagulation and fibrinolytic pathway. Also, it is established that the mutations in the genes of either pro-coagulant or anticoagulant are also associated the risk of VTE [12-15]. Chronic local or systemic inflammation occurs as the result of manifestation of various cardiovascular diseases including VTE. The pro-inflammatory molecules like cytokines and chemokines mediate this inflammation. However, the role of mutations in genes involved pro-inflammatory pathways is not well elucidated for VTE. There are new studies indicating that hypoxia is associated with inflammation [16,17]. The primary receptors of innate immunity are Toll-Like receptors (TLRs) and they can be a possible thread linking hypoxia and VTE by recognition of damage associated molecular patterns (DAMPs) generated by hypoxia. There are several lines of evidence showing TLRs links innate immune defence interaction with pro-inflammatory pathways that in turn affects the occurrence of diseases The various cells and tissues of cardiovascular system expresses TLRs on their surface [18]. There are various studies on SNPs analysis of TLRs conducted on western population that show that TLRs are involved in development and progression of diseases like atherosclerosis, thrombosis, congestive heart failure and cardiac dysfunction in sepsis etc. [19-22]. There are 11 TLRs that has been identified and cloned in humans. The number of studies related to different TLR polymorphism and their susceptibility to cardiovascular diseases is limited. The most widely studied TLR SNPs involved in CVDs are of TLR 2, 4 and 9 genes in western population. TLR2 A2258G (rs5743708) has been studied for association with Coronary restenosis [19], TLR4 A896G (rs4986790) for Atherosclerosis [20], and carotid artery atherogenesis [21] and TLR9 T-1237C (rs5743836) for Deep vein thrombosis, atherogenesis or restenosis [22].
The presence of SNP in a gene may influence or change the structure of the protein coded by that gene. In present study we aim to observe the effect of respective SNP on protein structure of TLR 2, 4 and 9 by using bioinformatics tool. The changes in protein structure will influence the recognition of signal by receptor and hence susceptibility to the disease. Furthermore, we also analysed association of single nucleotide polymorphism (SNP) in TLR genes with the risk of predisposing individuals to VTE by studying genetic polymorphisms in TLR2 (rs5743708), TLR4 (rs4986790) and TLR9 (rs5743836) genes in high altitude (HA) Thrombosis patients in comparison to healthy subjects.
Methods
Protein structure analysis
The protein structure of TLR2, TLR4 and TLR9 both wild and mutant harbouring SNP viz. TLR2 (rs5743708), TLR4 (rs4986790) and TLR9 (rs5743836) was modelled using Raptor X program [23]. Energy minimization was done using Swiss-Pdb Viewer [24]. Structures were visualized using PyMol(www.pymol.org). Structure validation was done using ERRAT (http://services.mbi.ucla.edu/ERRAT/), ProSA [25,26], PROCHECK [27] and PROVE. Surface topography and pockets were estimated using CASTp [28], for providing insights related to protein properties. Clefts and cavities were determined using ProFunc [29]. These regions are important from functional aspects. The area and volume of clefts are important for determining interacting residues, ligand and receptor binding. The mutants were generated using I-Mutant 3.0 providing details of their respective stabilities [31]. The DDG value less than zero is indicative of decrease in stability and more than zero specifies increase in stability. Channels in the protein structure were determined using beta cavityweb [31].
Study population
Twenty five male patients diagnosed with High Altitude induced VTE less than 45 years of age, hospitalized at the Department of Haematology, Army Hospital Research and Referral (AHRR), Delhi (a tertiary care unit), and forty healthy, age- and sex-matched volunteers were approached for consent to participate in the study. The medical history of all the participants under study was enquired. The exclusion criteria was followed to exclude participants with medical history of any risk for VTE either themselves or in their near relatives from the control group. For the subjects in patient group, documentation of clinical profile data along with date, and site of venous thrombosis (VT) episode along with the presence of predisposing factors (such as surgery, trauma, prolonged immobilization, hypertension, diabetes, familial history of bleeding) was done. The exclusion criteria for patient group were followed to exclude participants with a record of systemic diseases. The diagnosis of all the patients were confirmed by at least one of the radiological or neurological imaging methods like colour Doppler/contrast-enhanced computed tomography/computed tomography angiography/magnetic resonance imaging. The study was approved by the institutional human ethical committee of Defence Institute of Physiology & Allied Sciences (DIPAS) in line with ICMR guidelines.
Blood collection
Peripheral blood samples were collected from all subjects in vacutainer tubes (Becton and Dickinson, NJ, USA) having K2EDTA as anticoagulant. Blood collected was used for DNA isolation (to be used for genotyping analysis). The obtained DNA was stored at -20OC until used.
Genotyping
QIAamp DNA isolation kit (Qiagen, Germany), according to the manufacturer’s protocol was used for isolation of high-molecular-weight DNA from peripheral blood collected in EDTA vacutainers. To determine genomic DNA concentration NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) was used. DNA samples (100 ng/mL) were loaded on 1% agarose gel containing ethidium bromide and electrophoresed for half hour in order to be visualised under a gel imaging system (Fusion FX5, Vilber Lourmat, France) for the qualitative analysis of isolated DNA. Specific gene sequences for TLR2 A2258G (rs5743708), TLR4 A896G (rs4986790), and TLR9 T-1237C (rs5743836) regions were amplified using specific primer sequences. The final polymerase chain reaction (PCR) contained 100 ng of DNA, 10 pmol of each forward and reverse primer, and 1X RED Taq DNA polymerase buffer (Sigma Aldrich,US). The amplified PCR products were digested with specific restriction enzymes at their optimum temperature. The digested samples were loaded in 2-3 % agarose gel containing ethidium bromide and were subjected to gel electrophoresis to get band of different size. The gel electrophoresis experiments were performed at room temperature, and the bands were seen under UV. The allelic sizes of digested products were determined by comparing them with molecular-weight markers. The presence of specific alleles was confirmed by presence or absence of digested bands. Positive control and negative control i.e. SNP positive sample with known profile and SNP negative sample (blank) respectively were used in each experiment for verifying results. Complete details of PCR conditions and genotypes screened for different genes are listed in table 1.
Statistical analysisGenotypic and Allelic frequencies were determined by gene counting and compared by 2x2 contingency table. The statistical significance of differences between patients group and control group were estimated by chi square test, fisher’s exact test and calculation of odd’s ratio (with 95% confidence interval) using Graph Pad (Prism). Statistical significance criteria is p <0.05 for all tests.
| Table 1: Experimental details of PCR-RFLP conditions used. | ||||||
| Gene | SNP | Primer Details | PCR Product | Annealing Temp. | R.E. | Band Size (bp) |
| TLR2 | A2258G | F: TCCATTGAAAAGAGCCACAA R: GCCACTCCAGGTAGGTCTTG |
220bp | 56°C | PstI | 220 - AA 220, 184, 36 - GA 184, 36 - GG |
| TLR4 | A896G | F:GATTAGCATACTTAGACTACTACCTCCATG R: GATCAACTTCTGAAAAACGATTCCCAC |
249bp | 60°C | NcoI | 249 - AA 249, 223, 26 - AG 223, 26 - GG |
| TLR9 | T-1237C | F: ATGGGAGCAGAGACATAATGGA R: CTGCTTGCAGTTGACTGTGT |
135bp | 62°C | BstNB BstN1 | 108 - TT, 108, 60, 48 - TC 60, 48 - CC |
Result and Discussion
The concept that thrombosis can be linked with inflammation has been stated by Peter [32]. The fact that inflammation induces thrombosis is a well-established now but still its pathogenesis is unclear. The pro-inflammatory molecules like cytokines, chemokines links inflammation and hemostasis. A thrombotic state can occur in body due to inflammation as it tends to inhibit fibrinolytic activity along with natural anticoagulant pathways and increases pro-coagulant factors. The vessel endothelium is damaged by chronic inflammation that may cause loss of its vasodilatory properties, physiological anticoagulant, and anti-aggregatory effect. Also, venous thrombosis can be included by inflammation in absence of vessel wall damage [33-35].
TLRs are homologous family of pattern-recognition receptors that are differentially expressed among immune cells in mammals [36]. They initiate the signal transduction pathway by recognizing and binding to either conserved pathogen-associated molecular patterns (PAMPs) of microorganisms or damage-associated molecular patterns (DAMPs). After, trigger from TLRs there is activation of signal transduction pathways that lead to dendritic cell maturation and cytokine production [37]. TLRs play a crucial role in activation of the innate immunity [38]. There is growing number of evidences that emphasize on effect of specific single nucleotide polymorphisms (SNPs) in TLR genes, as a modulator of bacterial and viral infections. However, the exact relationship between inflammation, innate immunity, and thrombosis still lies infancy. To prove whether inflammation contributes to the onset or progression of thrombosis, we examined whether a constitutive defect in the innate immune response as occurs in the form of TLRs polymorphism was associated with reduced thrombotic risk or not. To assess this first we analysed the influence of respective SNP on protein structure of TLR2, TLR4 and TLR9 by in silico methods. This was followed by genotyping study to see their association with HA-induced thrombosis.
Influence of mutation on TLR2 protein structure
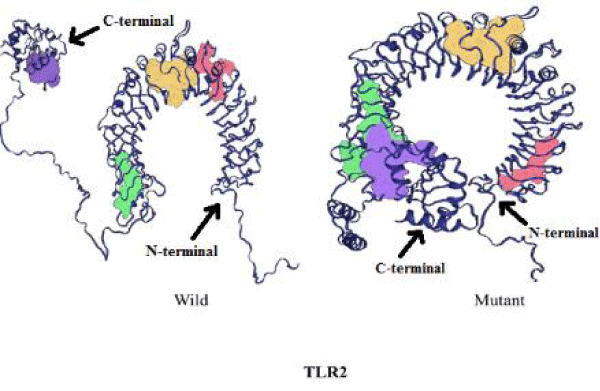
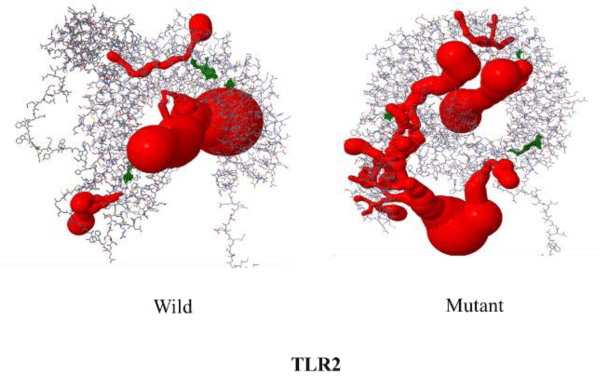
The SNP rs5743708 of TLR2 corresponds to Arg753Gln mutation in protein structure. The wild type TLR-2 contains 122 pockets which on mutating decreases to 119. The volume and surface area of the pockets increases on mutation (Table 2). The number of channels decreases from 15 in wild to 11 in the mutant protein (Table 3). The difference in free energy of the mutation (DDG) as calculated using I-Mutant is -1.38 Kcal/mol due to Arg753Gln in TLR2. The mutant is thermodynamically less stable than the wild TLR2 protein. This means mutant protein will transduce less signal downstream thereby making mutant phenotype protective for the given disease. The figures 1,2 depicts the change in structure of mutant and wild TLR2 protein.
| Table 2: Comparison of volume of 4 major pockets for TLR2 wild type and mutant protein. | ||
| Pockets | Wild type Protein (ų) |
Mutant protein (ų) |
| 1(in red) | 5264.58 | 14544.14 |
| 2(in violet) | 4639.36 | 7004.81 |
| 3(in yellow) | 2732.91 | 2009.39 |
| 4(in green) | 1436.91 | 1697.20 |
| Table 3: Comparison of channels and voids in TLR2. | |||
| Number of voids | Highest Void volume(ų) | Number of channels | |
| Wild | 66 | 23.535 | 15 |
| Mutant | 55 | 18.944 | 11 |
Influence of mutation on TLR4 protein structure
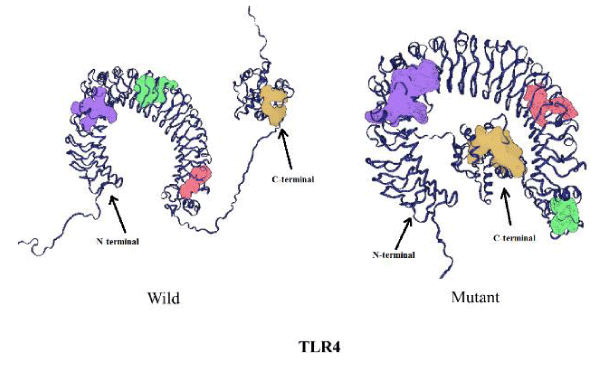
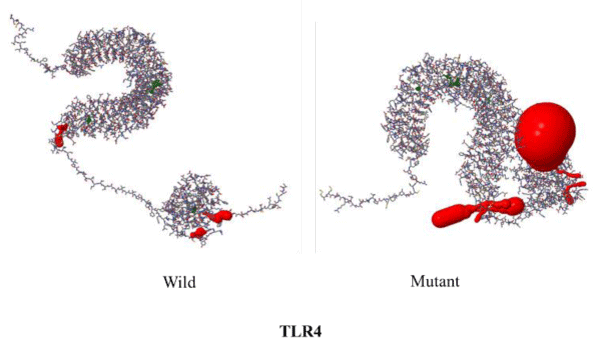
The mutation rs4986790 corresponds to Asp259Gly change in the protein and results in a decrease in the number of pockets from 119 in the wild type protein to 114 in the mutant (Table 4). However, there is no change observed in the number of channels on mutation. Only the surface area of the voids decreases on mutation (Table 5). The DDG value of the mutant corresponds to -0.92 Kcal/mol. I-Mutant results further predicted the less stability of the mutant with respect to the wild type protein. Based upon this data it can be hypothesized that mutant phenotype will be protective for disease because of less number of functional pockets as compared to wild type. Thus people harbouring mutant phenotype could be less susceptible to the disease. The changes in structure of mutant and wild TLR4 protein are shown in figures 3,4.
| Table 4: Comparison of channels and voids in TLR4. | |||
| Number of voids | Highest Void volume (ų) | Number of channels | |
| Wild | 85 | 25.538 | 3 |
| Mutant | 67 | 14.505 | 3 |
| Table 5: Comparison of volume of 4 major pockets for TLR4 wild type and mutant protein. | |||
| Pockets | Wild type Protein (ų) |
D299G (ų) |
T399I (ų) |
| 1(in red) | 3696.47 | 8634.94 | 4279.50 |
| 2(in violet) | 2906.30 | 2872.55 | 4454.16 |
| 3(in yellow) | 1921.22 | 884.67 | 2602.12 |
| 4(in green) | 2076.05 | 1280.81 | 830.25 |
Influence of mutation on TLR9 protein structure
The mutation rs5743836 is on the upstream region i.e. at T-1237C of the gene and it does not fall in open reading frame of the gene. Hence, this is not the part of protein coded by TLR9 gene and does not affect the protein structure.
Genotyping profiling
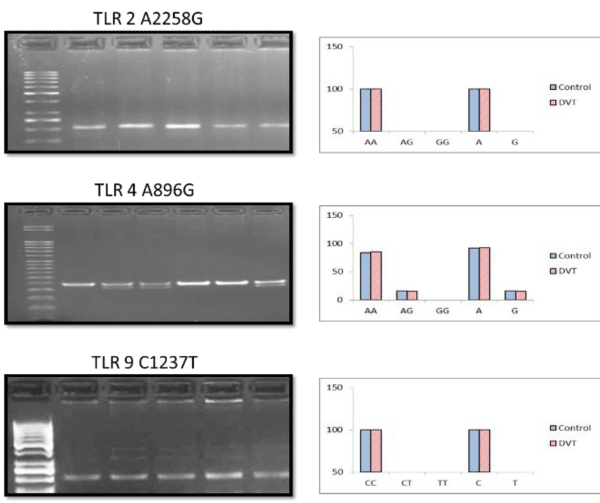
The genotyping profiling of SNPs under the study showed that TLR 2 and TRR9 genes are monomorphic in Indian population in both control and patient group. The frequency of occurrence of TLR4 SNP is also low. There is no homozygous mutant in both study groups. The frequency of AG (heterozygous mutant allele) is almost equal, 16% in patient and 15% in control group. This shows that these mutations are not prevailing in Indian population.
The TLR4 SNP Asp29Gly, under the study has been studied in Caucasian population. The studies reveal that it is associated with innate immunity related diseases, like chronic inflammatory disease and atherosclerosis [39,41]. The other group of researchers showed that in Caucasian populations TLR4 Asp299Gly polymorphism was associated with reductions in angiographic coronary artery disease, vascular inflammation, and clinical diabetes [42].
In contrary to this, the given SNP is very rarely reported in Asian ethnicity. The TLR4 Asp299Gly SNP was studied in 491 Han Chinese subjects but authors failed to detect any homozygous or heterozygous variant genotypes [43]. The association of ischemic stroke in ethnic Chinese patients with the TLR4 gene polymorphism was studied and almost no Asp299Gly variant was detected [44]. In the present study also there was no association seen between the given polymorphism and thrombosis in Indian population.
The TLR9-1237T/C variant has been studied in Korean and Japanese population. In Korean population low frequency (<0.3%) of the variant was observed [45], whereas in a Japanese population no TLR9-1237T/C polymorphism was present in 183 patients with SLE and 198 controls [46]. It has been also reported that, Chinese population also have much less variations at position-1237, our result is in accordance of these studies as we did not detect any SNP at given position in Indian population [47].
The details of allelic frequencies of 3 SNPs are given in figure 5 (Table 6).
Figure 5: Graphical representation of Genotypic and Allelic frequencies along with representative gel images for the 3 SNPs under study.
| Table 6: Statistical comparison of the two groups with ᵪ2 test, fisher’s exact, odd’s ratio & confidence interval. | |||||
| Gene Polymorphism | Pearson’s Chi square | Fisher’s exact | |||
| χ2 (df=2) | P-value | P-value | Odd’s ratio | CI | |
| TLR4 (A896G) | 0.012 | 0.9134 | 1.00 | 1.0794 | 95% |
Conclusion
The function of a protein is greatly influenced by its structure. Any change in the structure may influence the functionality of the given protein. Thus, the changes in protein structure due to presence of SNP in the corresponding gene may alter its function. Guided by this hypothesis we tried to see the effect of SNPs of TLR2 and TLR4 genes on their protein structure. In both TLR2 and TLR4 the mutant proteins harbour less number of pockets as compared to wild. The cavities and voids are important for ligand binding function in proteins. Many small ligands including therapeutic molecules bind in these cavities. Thus, increase in the number or cavities results in a corresponding increase in the surface area of cavities leading to enhanced ligand binding and vice versa. And, decrease in number of binding pockets will result in reduced downstream signal and hence less aggravated response. The results suggest that these mutations can be protective as they decrease number of binding pockets. Thus, based upon above data we designed further studies on genotypic level by using PCR RFLP approach.
Genotyping studies showed that there is no statistically significant difference between the controls and the patient group in polymorphism of TLR4 gene. This indicates that the frequency of occurrence of these mutations is highly low in Indian population. Also, the population size under the study is small due to less availability of HA-VTE patient samples. Therefore, due to paucity of samples, no association could be established between TLR polymorphism and the disease under present study. But in silico results are indicative of SNPs being protective and associated with decreased susceptibility to disease. Therefore, it can be hypothesized that mutant phenotype if present in population could lead to less susceptibility for the VTE. This leaves the wide scope of study that need to be carried out on genetic level in larger population size.
Acknowledgment
The authors are extremely thankful to all the volunteers who participated in the study. We are grateful to Directorate General Armed Forces Medical Sciences and Scientist, staff, research scholars of the institute who cooperated and provided logistic support in management and collection of healthy and patient blood samples. We are also thankful to Dr. Zahid Ashraf, JMI, Delhi India for his support during the study.
References
- Homans J. Thrombosis of the deep leg veins due to prolonged sitting. N Engl J Med. 1954; 250: 148-149. Ref.: https://goo.gl/za8FH9
- Gibbs NM. Venous thrombosis of the lower limbs with particular to bed rest. Br J Surg. 1957; 45: 209-236. Ref.: https://goo.gl/t2j1EN
- Hull RD, Raskob GE. Prophylaxis of venous thromboembolic disease following hip and knee surgery. J Bone Joint Surg Am.1986; 68: 146-150. Ref.: https://goo.gl/RN48ra
- Ward M. Mountain Medicine: A Clinical Study of Cold and High Altitude. 1975; Ref.: https://goo.gl/dDt4Ab
- Zangari M, Fink L, Tolomelli G, Lee JC, Stein BL, et al. Could hypoxia increase the prevalence of thrombotic complications in polycythemia vera? Blood Coagul Fibrinolysis. 2013; 24: 311-316. Ref.: https://goo.gl/8UkEK6
- Anand AC, Saha A, Kumar R, Sharma V, Jha SK. Portal system thrombosis: a new dimension of high altitude illnesses. Trop Gastroenterol. 2000; 21: 172-173. Ref.: https://goo.gl/FrQesU
- Cheng S, Chng SM, Singh R. Cerebral venous infarction during a high altitude expedition. Singapore Med J. 2009; 50: e306-308. Ref.: https://goo.gl/Txhhdz
- Gupta N, Ashraf MZ. Exposure to high altitude: a risk factor for venous thromboembolism? Semin Thromb Hemost. 2012; 38: 156-163. Ref.: https://goo.gl/MdT1w6
- Anand AC, Jha SK, Saha A, Sharma V, Adya CM. Thrombosis as a complication of extended stay at high altitude. Natl Med J India. 2001; 14: 197-201 Ref.: https://goo.gl/3pLNJg
- White RH, Zhou H, Romano PS. Incidence of idiopathic deep vein thrombosis and secondary thromboembolism among ethnic groups in California. Ann Inter Med. 1998; 128: 737-740. Ref.: https://goo.gl/vuUKa1
- Kapoor VK. Venous thromboembolism in India. Editorials. Natl Med J India. 2010; 23: 93-95.
- Griffin JH, Evatt B, Zimmerman TS. Deficiency of protein C in congenital thrombotic disease. J Clin Invest. 1981; 68: 1370-1373. Ref.: https://goo.gl/FxEHjB
- Koster T, Rosendaal FR, Briët E, van der Meer FJ, Colly LP, et al. Protein C deficiency in a controlled series of unselected outpatients: an infrequent but clear risk factor for venous thrombosis (Leiden Thrombophilia Study). Blood. 1995; 85: 2756-2761. Ref.: https://goo.gl/fFLbzx
- Bezemer ID, Rosendaal FR. Predictive genetic variants for venous thrombosis: what’s new? Semin Hematol. 2007; 44: 85-92. Ref.: https://goo.gl/khg4Q2
- Heit JA, Armasu SM, Asmann YW, Cunningham JM, Matsumoto ME, et al. A genome-wide association study of venous thromboembolism identifies risk variants in chromosomes 1q24.2 and 9q. J Thromb Haemost. 2012; 10: 1521-1531. Ref.: https://goo.gl/Qswtwx
- Mannucci PM, Gringeri A, Peyvandi F, Di Paolantonio T, Mariani G. Short-term exposure to high altitude causes coagulation activation and inhibits fibrinolysis. Thromb Haemost. 2002; 87: 342-343. Ref.: https://goo.gl/Asrhok
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011; 364: 656-665. Ref.: https://goo.gl/ea8Ncw
- Frantz S, Ertl G, Bauersachs J. Mechanism of disease: Toll Like Receptors in cardiovascular diseases. Nat Clin Pract Cardiovasc Med. 2007; 4: 444–454. Ref.: https://goo.gl/JfJP2m
- Hamann L, Gomma A, Schröder NW, Stamme C, Glaeser C, et al. A frequent Toll like receptor (TLR) 2 polymorphism is a risk factor for coronary restenosis. J Mol Med. 2005; 83: 478–485. Ref.: https://goo.gl/LLYXTW
- Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, et al. Toll-like receptor 4 polymorphism and atherogenesis. N Engl J Med. 2002; 347:185–192. Ref.: https://goo.gl/o3xEaa
- Hodgkinson CP1, Ye S. Toll-like receptors, their ligands, and atherosclerosis. Sci World J. 2011; 11: 437–453. Ref.: https://goo.gl/K62h2M
- Hamann L, Glaeser C, Hamprecht A, Gross M, Gomma A, et al. Toll-like receptor (TLR)-9 promotor polymorphisms and atherosclerosis. Clin Chim Acta. 2006; 364: 303–307. Ref.: https://goo.gl/LorUDw
- Källberg M, Wang H, Wang S, Peng J, Wang Z, et al. Template-based protein structure modeling using the RaptorX web server. Nature Protocols. 2012; 7: 1511–1522. Ref.: https://goo.gl/GLBAzG
- Källberg M, Wang H, Wang S, Peng J, Wang Z, et al. Template-based protein structure modeling using the RaptorX web server. Nature Protocols. 2012; 7: 1511–1522. Ref.: https://goo.gl/fuefUb
- Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Research. 2007; 35: W407-W410. Ref.: https://goo.gl/57YcWa
- Sippl MJ. Recognition of Errors in Three-Dimensional Structures of Proteins. Proteins. 1993; 17: 355-362. Ref.: https://goo.gl/EuZEie
- Laskowski RA, Moss DS, Thornton JM. Main-chain bond lengths and bondangles in protein structures. J Mol Biol. 1993; 231: 1049–1067. Ref.: https://goo.gl/csfJh2
- Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, et al. CASTp:computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res 2006, 34(Web Server issue): W116–W118. Ref.: https://goo.gl/F8n6Qn
- Laskowski RA, Watson JD, Thornton JM. ProFunc: a server for predicting protein function from 3D structure. Nucleic Acids Res. 2005; 33 (Web Server issue): W89–W93. Ref.: https://goo.gl/q6AzY5
- Capriotti E, Calabrese R, Casadio R. Predicting the insurgence of human genetic diseases associated to single point protein mutations with support vector machines and evolutionary information. Bioinformatics. 2006; 22: 2729–2734. Ref.: https://goo.gl/SU8AC5
- Kim JK, Cho Y, Lee M, Laskowski RA, Ryu SE, et al. BetaCavityWeb: a webserver for molecular voids and channels. Nucleic Acids Res. 2015; W413-418. Ref.: https://goo.gl/hthiKx
- Libby P, Simon DI. Inflammation and Thrombosis. The Clot Thickens. Circulation. 2001; 103: 1718-1720. Ref.: https://goo.gl/pWiZgg
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002; 105: 1135–1143. Ref.: https://goo.gl/j5Ym2X
- Zebrack JS, Anderson JL. The role of inflammation and infection in the pathogenesis and evolution of coronary artery disease. Current Cardiology Reports. 2002; 4: 278–288. Ref.: https://goo.gl/pcjdUZ
- Liu F, Lu W, Qian Q, Qi W, Hu J, et al. Frequency of TLR 2, 4, and 9 Gene Polymorphisms in Chinese Population and Their Susceptibility to Type 2 Diabetes and Coronary Artery Disease. J Biomed Biotechnol. 2012; 2012: Article ID 373945. Ref.: https://goo.gl/CHHgq3
- Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature. 1997; 388: 394–397. Ref.: https://goo.gl/8FS3AK
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124: 783–801. Ref.: https://goo.gl/eyckzd
- Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med (Berl). 2006; 84: 712–725. Ref.: https://goo.gl/2gN9rC
- Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, et al. Toll-like receptor 4 polymorphisms and atherogenesis. The New England Journal of Medicinel. 2002; 347: 185–192. Ref.: https://goo.gl/hujrpw
- Hollestelle SC, De Vries MR, Van Keulen JK, Schoneveld AH, Vink A, et al. Toll-like receptor 4 is involved in outward arterial remodeling. Circulation. 2004; 109: 393–398. Ref.: https://goo.gl/NcqXx1
- Boekholdt SM, Agema WR, Peters RJ, Zwinderman AH, van der Wall EE, et al. Variants of toll-like receptor 4 modify the efficacy of statin therapy and the risk of cardiovascular events. Circulation. 2003; 107: 2416–2421. Ref.: https://goo.gl/RLGTh4
- Kolek MJ, Carlquist JF, Muhlestein JB, Whiting BM, Horne BD, et al. Toll-like receptor 4 gene Asp299Gly polymorphism is associated with reductions in vascular inflammation, angiographic coronary artery disease, and clinical diabetes. Am Heart J. 2004; 148: 1034–1040. Ref.: https://goo.gl/vqUa9r
- Hang J, Zhou W, Zhang H, Sun B, Dai H, et al. TLR4 Asp299Gly and Thr399Ile polymorphims are very rare in the Chinese population. J Endotoxin Res. 2004; 10: 238–240. Ref.: https://goo.gl/jiwGNY
- Lin YC, Chang YM, Yu JM, Yen JH, Chang JG, et al. Toll-like receptor 4 gene C119A but not Asp299Gly polymorphism is associated with ischemic stroke among ethnic Chinese in Taiwan. Atherosclerosis. 2005; 180: 305–309. Ref.: https://goo.gl/TTi92x
- Hur JW, Shin HD, Park BL, Kim LH, Kim SY, et al. Association study of Toll-like receptor 9 gene polymorphism in Korean patients with systemic lupus erythematosus. Tissue Antigens. 2005; 65: 266–270. Ref.: https://goo.gl/nD33Eq
- Tao K, Fujii M, Tsukumo SI, Maekawa Y, Kishihara K, et al. Genetic variations of Toll-like receptor 9 predispose to systemic lupus erythematosus in Japanese population. Annals of the Rheumatic Diseases. 2007; 66: 905–909. Ref.: https://goo.gl/gkwdsb
- Ng MW, Lau CS, Chan TM, Wong WH, Lau YL. Polymorphisms of the toll-like receptor 9 (TLR9) gene with systemic lupus erythematosus in Chinese. Rheumatology. 2005; 44: 1456–1457. Ref.: https://goo.gl/NoayCf